1200 - 1000 - 800 - energy (zJ) 600 - -B 400 - A 200 - Use this diagram to complete the table below. What is the energy of the electron in the ground state? 250 zJ Ox10 What is the energy of the electron in the first excited state? ? 550 zJ If the electron makes the transition shown by the red arrow, from C to A, will a photon be absorbed or O absorbed emitted? O emitted Calculate the wavelength of the photon that would be absorbed or emitted. Round your answer to 3 significant digits. nm O
1200 - 1000 - 800 - energy (zJ) 600 - -B 400 - A 200 - Use this diagram to complete the table below. What is the energy of the electron in the ground state? 250 zJ Ox10 What is the energy of the electron in the first excited state? ? 550 zJ If the electron makes the transition shown by the red arrow, from C to A, will a photon be absorbed or O absorbed emitted? O emitted Calculate the wavelength of the photon that would be absorbed or emitted. Round your answer to 3 significant digits. nm O
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 39P: Chapter 3 introduced the concept of a double bond between carbon atoms, represented by C=C , with a...
Related questions
Question
pllease answer quicklyy thank you, i believe the rest is correct not sure how to do the math for the 4th one
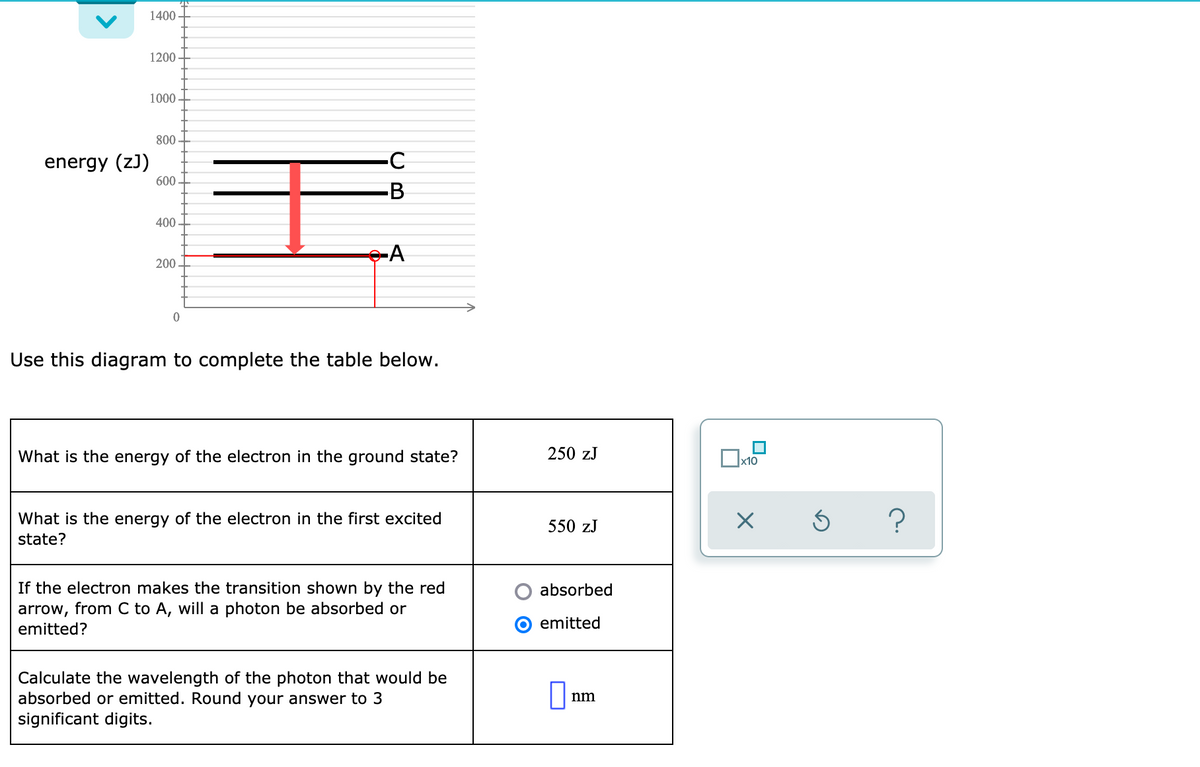
Transcribed Image Text:1400
1200
1000
800
energy (z)
600
-B
400
200
Use this diagram to complete the table below.
What is the energy of the electron in the ground state?
250 zJ
x10
What is the energy of the electron in the first excited
550 zJ
state?
If the electron makes the transition shown by the red
arrow, from C to A, will a photon be absorbed or
emitted?
absorbed
emitted
Calculate the wavelength of the photon that would be
absorbed or emitted. Round your answer to 3
significant digits.
nm
<>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
