2. The molecule buta-1,3-diene is an easily isolatable gas with a normal boiling point just below the freezing point of ice. Cyclobuta-1,3-diene, however, is a very unstable molecule that can only be isolated below 35 K (-238 °C). a. (1) Draw two molecular orbital (MO) diagrams, one for (a) cyclobutadiene and one for (b) buta-1,3-diene. For each energy level in each diagram, (ii) draw a p-orbital representation of the MO that corresponds to that energy level. Be sure to (ii) shade your orbitals appropriately and (iv) draw any and all nodes. Next, (v) label each orbital as bonding, non-bonding, or anti-bonding and briefly (vi) explain how you made those determinations. Finally, (vii) show how many electrons fill each orbital. b. Using the MO diagrams you just prepared, (i) explain why there is such a difference in stability between the two compounds buta-1,3-diene and cyclobuta-1,3-diene. As part of your answer, be sure to (i) explain why cyclo-1,3- butadiene cannot exist at room temperature
2. The molecule buta-1,3-diene is an easily isolatable gas with a normal boiling point just below the freezing point of ice. Cyclobuta-1,3-diene, however, is a very unstable molecule that can only be isolated below 35 K (-238 °C). a. (1) Draw two molecular orbital (MO) diagrams, one for (a) cyclobutadiene and one for (b) buta-1,3-diene. For each energy level in each diagram, (ii) draw a p-orbital representation of the MO that corresponds to that energy level. Be sure to (ii) shade your orbitals appropriately and (iv) draw any and all nodes. Next, (v) label each orbital as bonding, non-bonding, or anti-bonding and briefly (vi) explain how you made those determinations. Finally, (vii) show how many electrons fill each orbital. b. Using the MO diagrams you just prepared, (i) explain why there is such a difference in stability between the two compounds buta-1,3-diene and cyclobuta-1,3-diene. As part of your answer, be sure to (i) explain why cyclo-1,3- butadiene cannot exist at room temperature
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter18: Aromaticity
Section: Chapter Questions
Problem 26CTQ
Related questions
Question
help please
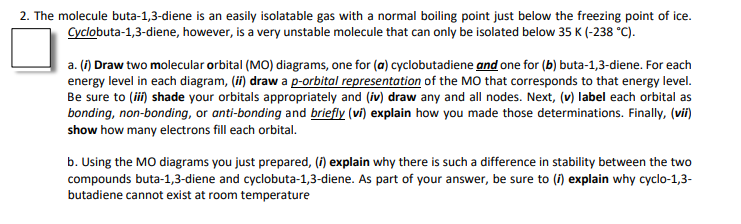
Transcribed Image Text:2. The molecule buta-1,3-diene is an easily isolatable gas with a normal boiling point just below the freezing point of ice.
Cyclobuta-1,3-diene, however, is a very unstable molecule that can only be isolated below 35 K (-238 °C).
a. (1) Draw two molecular orbital (MO) diagrams, one for (a) cyclobutadiene and one for (b) buta-1,3-diene. For each
energy level in each diagram, (ii) draw a p-orbital representation of the MO that corresponds to that energy level.
Be sure to (ii) shade your orbitals appropriately and (iv) draw any and all nodes. Next, (v) label each orbital as
bonding, non-bonding, or anti-bonding and briefly (vi) explain how you made those determinations. Finally, (vii)
show how many electrons fill each orbital.
b. Using the MO diagrams you just prepared, (i) explain why there is such a difference in stability between the two
compounds buta-1,3-diene and cyclobuta-1,3-diene. As part of your answer, be sure to (f) explain why cyclo-1,3-
butadiene cannot exist at room temperature
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning