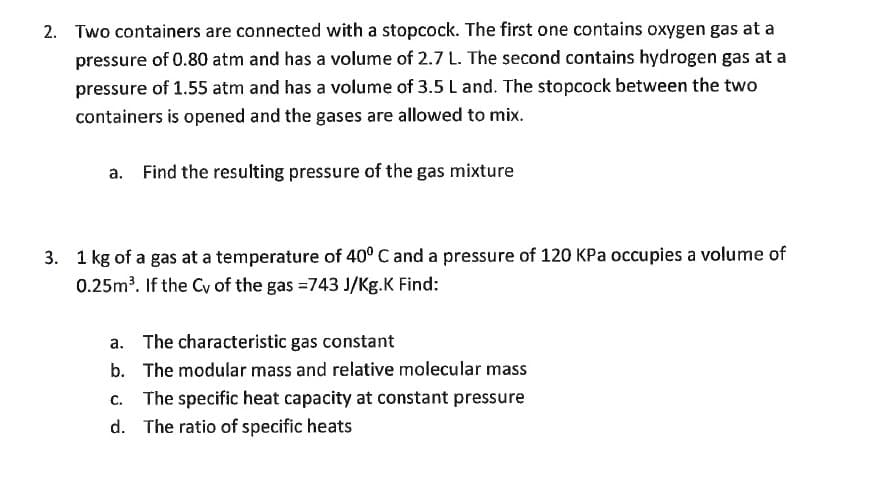
2. Two containers are connected with a stopcock. The first one contains oxygen gas at a pressure of 0.80 atm and has a volume of 2.7 L. The second contains hydrogen gas at a pressure of 1.55 atm and has a volume of 3.5 L and. The stopcock between the two containers is opened and the gases are allowed to mix. a. Find the resulting pressure of the gas mixture
2. Two containers are connected with a stopcock. The first one contains oxygen gas at a pressure of 0.80 atm and has a volume of 2.7 L. The second contains hydrogen gas at a pressure of 1.55 atm and has a volume of 3.5 L and. The stopcock between the two containers is opened and the gases are allowed to mix. a. Find the resulting pressure of the gas mixture
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter20: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 20.4OQ
Related questions
Question
Question attached about Latent Heat and
Transcribed Image Text:2. Two containers are connected with a stopcock. The first one contains oxygen gas at a
pressure of 0.80 atm and has a volume of 2.7 L. The second contains hydrogen gas at a
pressure of 1.55 atm and has a volume of 3.5 L and. The stopcock between the two
containers is opened and the gases are allowed to mix.
a. Find the resulting pressure of the gas mixture
3. 1 kg of a gas at a temperature of 40° C and a pressure of 120 KPa occupies a volume of
0.25m³. If the Cy of the gas =743 J/Kg.K Find:
a. The characteristic gas constant
b. The modular mass and relative molecular mass
c. The specific heat capacity at constant pressure
d. The ratio of specific heats

Transcribed Image Text:3. A student added lumps of ice to cool a Lemonade. He discovers that 80g of ice at a
temperature of 0°C cools 0.25 kg of lemonade from 25°C to 6°C. The latent heat of fusion of
ice is 0.33 MJ/kg and the specific heat capacity of water is 4.2 kJ/kg K. Determine:
a. The energy gained by the ice in melting
b. The energy gained by the melted ice
c. The energy lost by the lemonade
d. A value for the specific heat capacity of the lemonade
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 19 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning