2. Using Excel, make a graph of the following data. Plot Mass vs. Volume for the following data on the same graph. Use a linear fit of each set of data and include the equation of the best fit line (2 fits and 2 equations, making sure you can tell which equation is for which set of data). Submit your graph and answer the three questions below. Volume (ml) 5.00 10.00 15.00 20.00 30.00 Mass of Al (g) 43.195 56.053 70.961 82.499 108.988 Mass of Zr (g) 63.797 94.708 127.933 158.580 227.037 For both straight lines, the slope is essentially equal to the density of the element. The y-intercept is equal to the mass of the beaker that held the substance. Answer the following questions. a) For each element, what is the density (slope)? What is the mass of the beaker (y-intercept)? Be sure to include units. b) If you have 120.000 g of Al and beaker, what is the volume? $24.0 c) When the volume of Zr is 25.00 mL, what is the mass?
2. Using Excel, make a graph of the following data. Plot Mass vs. Volume for the following data on the same graph. Use a linear fit of each set of data and include the equation of the best fit line (2 fits and 2 equations, making sure you can tell which equation is for which set of data). Submit your graph and answer the three questions below. Volume (ml) 5.00 10.00 15.00 20.00 30.00 Mass of Al (g) 43.195 56.053 70.961 82.499 108.988 Mass of Zr (g) 63.797 94.708 127.933 158.580 227.037 For both straight lines, the slope is essentially equal to the density of the element. The y-intercept is equal to the mass of the beaker that held the substance. Answer the following questions. a) For each element, what is the density (slope)? What is the mass of the beaker (y-intercept)? Be sure to include units. b) If you have 120.000 g of Al and beaker, what is the volume? $24.0 c) When the volume of Zr is 25.00 mL, what is the mass?
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter3: Measurement And Chemical Calculations
Section: Chapter Questions
Problem 99E
Related questions
Question
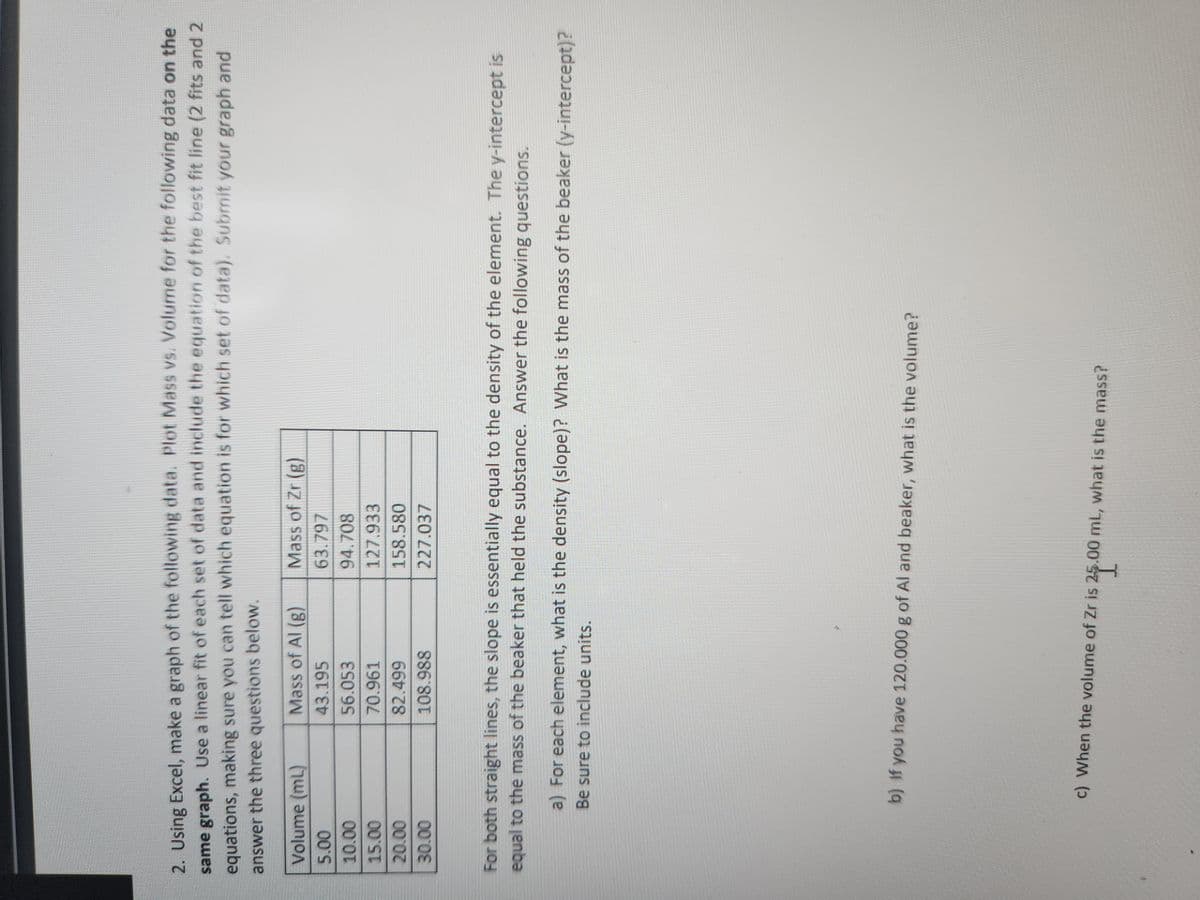
Transcribed Image Text:2. Using Excel, make a graph of the following data. Plot Mass vs. Volume for the following data on the
same graph. Use a linear fit of each set of data and include the equation of the best fit line (2 fits and 2
equations, making sure you can tell which equation is for which set of data). Submit your graph and
answer the three questions below.
Volume (ml)
5.00
10.00
15.00
20.00
30.00
Mass of Al (g)
43.195
56.053
70.961
82.499
108.988
Mass of Zr (g)
63.797
94.708
127.933
158.580
227.037
For both straight lines, the slope is essentially equal to the density of the element. The y-intercept is
equal to the mass of the beaker that held the substance. Answer the following questions.
a) For each element, what is the density (slope)? What is the mass of the beaker (y-intercept)?
Be sure to include units.
b) If you have 120.000 g of Al and beaker, what is the volume?
21.⁰
c) When the volume of Zr is 25.00 mL, what is the mass?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning



Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


