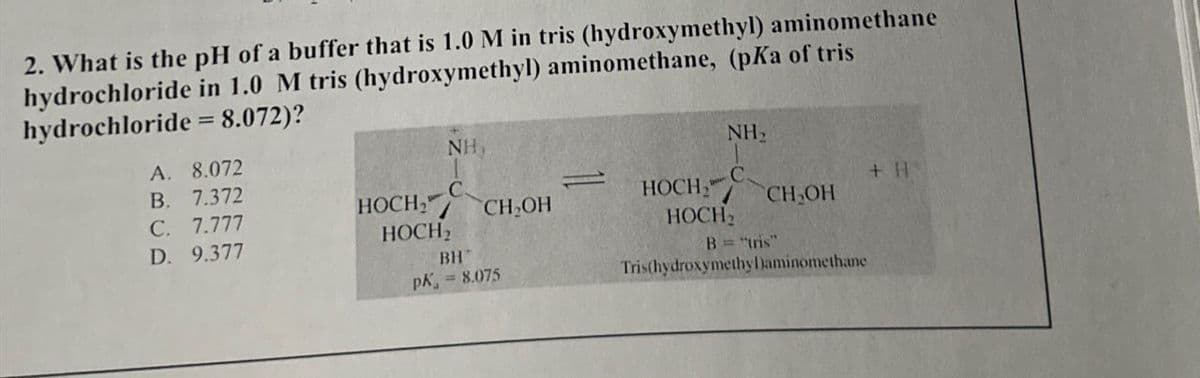
2. What is the pH of a buffer that is 1.0 M in tris (hydroxymethyl) aminomethane hydrochloride in 1.0 M tris (hydroxymethyl) aminomethane, (pKa of tris hydrochloride = 8.072)? NH NH₂ A. 8.072 B. 7.372 C HOCH," HOCH + H C. 7.777 CH₂OH CH₂OH HOCH2 HOCH D. 9.377 BH B="tris" PK = 8.075 Tris(hydroxymethy Daminomethane
Q: 4NH3+502 → 4NO + 6H20 The standard enthalpies of formation for NH3, NO and H2O are 294.1 kj/mol,…
A:
Q: Please answer the following chem question
A:
Q: Consider the reaction N2(g)+202(g) >2NO2(g). Use the standard thermodynamic data in the tables…
A: Please comment down for any doubt. I hope my answer helps you.
Q: Consider the following system at equilibrium where K = 1.80×102 and AH° = 10.4 kJ/mol at 698 K. 2 HI…
A: First Question: 1.) TRUE. The enthalpy change (ΔH) of the reaction is positive, which indicates that…
Q: What is the chemistry of how spf blocks uv rays from the sun Please please please answer as fast as…
A: Sunscreen works by shielding the skin from the harmful effects of ultraviolet (UV) radiation, which…
Q: 13 Rank the following in order of increasing Bronsted acidity (lowest to highest) A. B. es g C. OB<…
A: Based on the image you sent, it appears to be a schematic for ranking the following in order of…
Q: Propose a chemical structure for the name below. Make sure to clearly define the stereochemistry…
A:
Q: = 1.6 x 10-10 Given the reactions below, calculate the molar solubility of AgCl (s) in 0.566 M NH3…
A: Step 1: Step 2:There is 0.566 instead of 0.655 by mistake I solved this acc to 0.655 ... Hope you…
Q: 1. Assuming the validity of Raoult's law, do the following calculations for the benzene/toluene…
A:
Q: Help calculate.
A: Step 1: Step 2: Step 3: Step 4:
Q: Identify all the products expected when the following compound is treated with aqueous acid. N- N…
A:
Q: 6. Determine AG (in kJ mol¹ to one decimal place) for the following balanced reaction at 50 °C when…
A: Step 1:Relation between ΔG and ΔGo isΔG=ΔGo+RTlnQ...(1)T is temperature in kelvin.Q is reaction…
Q: 49.4 g of CaCO3 (s) reacts with 878 mL 1.8 M HCI (aq). Theoretically, how many grams of CO2 gas can…
A: Detailed explanation:Step 1: Write the balanced chemical equationHere's what happens when…
Q: *Reaction is in image* Questions based on reaction in image: The oxidizing agent is: The reducing…
A: Step 1: Determine the oxidation states of all the atoms in the equation. For Cl2, the charge is 0…
Q: *Reaction is in image* Questions based on reaction in image: The oxidizing agent is: The reducing…
A: When one species loses the electrons increasing its oxidation number is called oxidized and the…
Q: How many protons are present in Pb-206? How many neutrons are present in 17N? Give text symbol of…
A: Use a periodic table to see the values1. Atomic Number = ProtonsPb atomic number is 82Protons=822.…
Q: CIO (aq) + Cr(OH)3(s) CrO42(aq) + Cl¯(aq) basic What is the standard cell potential, E° cell, for…
A: Step 1:The standard electrode potential, often referred to as standard potential, indicates the…
Q: For each of the following reactions give the Major product(s) of the reaction only and statethe…
A: Refer to the boxed structures below for the structures of the major products: C) The mechanism below…
Q: Provide the major products, including steps
A: a) The Jones reagent is a typical oxidizing solution prepared by dissolving CrO3 in aqueous H2SO4.…
Q: please answer in text form and in proper format answer with must explanation , calculation for each…
A: Given: 298KA. The cell is under standard conditions.B.[] = 3.0 M[] = 0.010 M[] = 0.10 MC.[] = 0.010…
Q: The corrected cell potential for the following galvanic cell was determined to be (1.62x10^0) V.…
A:
Q: A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown:…
A: Given:Zn2+ (aq) + 2e- <---> Zn (s) -0.76 volt Cd2+ (aq) + 2e-…
Q: How does a decrease in the temperature of a chemical reaction affect the reaction rate? The reaction…
A: Step 1: How Decreasing Temperature Lowers Reaction RatesChemical reactions occur when reactant…
Q: None
A: The reactant is an organic molecule with an amine (NH2) group and a ketone (C=O) group on adjacent…
Q: Predict the products for the following reactions. 1. NaOH 2. 3. H3O+ О 1. CH3MgBr 2. H3O+ 1.…
A: The text in the image refers to a spring with constant k attached to a mass m on a frictionless rod.…
Q: Draw the structure(s) of the major organic product(s), including counterions, of the following…
A:
Q: Question 5 Which set of Newman projections represents correct the most stable and the least stable…
A: Question 5: The most stable is when they CH3 are anti to each other180∘. Whereas the least stable…
Q: please add missing product
A: para product will be more stable
Q: n What would be the expected result from the following reaction? (cy is an abbreviation of…
A: Step 1: The expected result from the following reaction is So, pentanal is the correct answer.
Q: None
A: Step name of the compound is "2-bromo-7-methyloctan-3-one" Steps followed while naming are :- 1.…
Q: Can you write down retrosynthesis mecanism and show pilar Br افي ง
A: Step 1: ** Bromobenzene's Retrosynthesis : *** Target Molecule : C6H5Br (bromobenzene). * Functional…
Q: Draaw the starting structure that would lead to the major product shown under the provided…
A:
Q: The following shows 1.00L bulbs connected by valves. Each bulb contains neon gas with amounts…
A: Find first the pressure in bulb C.using the ration if bulb A's gas atoms to bulb C's gas atoms.…
Q: Describe the three main mechanisms for transport across membranes?
A: Reference:https://life.nthu.edu.tw/~b830473/transmechanism.htmlhttps://www.inspiritvr.com/mechanisms…
Q: Demonstrate that the probability of occupying a given vibrational energy level does not depend on…
A: Step 1:Step 2:Step 3:Step 4:
Q: Please don't provide handwritten solution ..
A: In organic chemistry, the acid-catalyzed hydration of an alkene to form an alcohol is a fundamental…
Q: Using these metal ion/metal standard reduction potentials Cd2+(aq)|Cd(s)…
A: Standard reduction potentialsCd2+ + 2e- → Cd E˚ = -0.40 VCu2+ + 2e- → Cu…
Q: Question 9
A: 1. To find the total number of valence electrons, let's add up the valence electrons of each atom:…
Q: Which substituent is a deactivating ortho/para director? -CO₂Et -N(CH3)3 -OH T -SO3H O a. I O b. ll…
A: thus , answer is option (e.) V
Q: (b) For each of the following isotopes, identify the probable mode of decay and construct a balanced…
A: Plutonium-239 (Pu-239) undergoes fission. Fission is a nuclear decay process where a heavy nucleus…
Q: 1. A pure solvent freezes at 6.2oC. Determine the molar mass of a 0.8712 g nonelectrolyte dissolved…
A: Step 1: Step 2: Step 3: Step 4:
Q: What is the solubility of Fe(OH)₂ at a pH of 11.00? (Ksp Fe(OH)₂ is 4.9 × 10⁻¹⁷)
A: Step 1: To determine the solubility of Fe(OH)2 at a pH of 11.00, we need to consider the following…
Q: How many protons are in the Pb-208 isotope? 126 208 82 290
A: A. How many protons are in Pb-208 isotope?-Pb corresponds to element Lead(Pb).-Pb on the periodic…
Q: Calculate the G°rxn using the following information. 2 HNO3(aq) +NO(g) → 3 NO2(g) + H2O(l) AG'rxn =?…
A:
Q: How many grams of water can be heated from 20.00C to 78.00C using 12500.0 J? The specific heat…
A: GUIDE: (1) Heat Energy Formula Q=mc(Tf−Ti)where:Q = energy m = mass of the substancec = specific…
Q: Please answer these questions!!
A: Sure! Let's solve these quantum mechanics problems step by step: Problem 1: Average Position (z) for…
Q: The wow expert Hand written solution is not allowed please
A: Step 1:Given, decomposition reaction is2 NOBr (g) ⇌ 2 NO (g) + 1 Br2 (g) Step 2:Kp is an equilibrium…
Q: In the laboratory a student finds that it takes 16.5 Joules to increase the temperature of 10.5…
A: Step 1: Given DataQ = 16.5 JouleT1 = 22.6 °CT2 = 35.3 °Cm = 10.5 gramsStep 2: Temperature…
Q: Show the products of the following reactions
A:
Q: Predict the missing product of this organic reaction please:
A:
Step by step
Solved in 2 steps

- What will be the appropriate pH of a buffer prepared by mixing 100 ml of 0.1 M Na2HPO4 and 100 ml of 0.1 M NaH2PO4? (For H3PO4, pKa1 = 2.1; pKa2 = 6.8; pKa3 = 12.5)(5) a) A nitrous acid/sodium nitrite buffer solution is prepared. 20.0 mL of 6.50 M nitrous acid (HNO2) and 8.52 g of sodium nitrite (NaNO2, molar mass 68.99 g/mol) are placed in a 250. mL volumetric flask. The flask is filled to the mark with water and inverted 10 times to mix thoroughly. What is the pH of this buffer? Take Ka for Nitrous Acid to be 4.52 x 10-4. pH: b) 2.00 mL of a 2.50 M solution of NaOH is added to 75.0 mL of the buffer prepared above. What is the pH of this solution? How much did the pH change? pH: change in pH: c) What is the pH of pure distilled water (just give the pH, no calculations are necessary). d) If 2.00 mL of 2.50 M…A solution is made by dissolving 0.1283 g of the acid form of the buffer TRIS (molecular mass = 157.60 g/mol) along with 0.1975 g of the basic form of TRIS (molecular mass = 121.136 g/mol). The pK a of TRIS is 8.08. A very small amount of potassium dihydrogen phosphate is added (not enough to cause any change in the pH). What is the fractional amount of the potassium dihydrogen phosphate that is in the PO 4 3- form? Phosphoric acid is a triprotic acid with pK a1 = 2.16, pK a2 = 7.21 and pK a3 = 12.32.
- You make a 200mM potassium phosphate buffer (pKa of 7.20) with monobasic potassium phosphate (molecular weight 136.09 g/mol) and dibasic potassium phosphate (174.20 g/mol). For 100mL of buffer, at a pH of 7.20, how many grams of monobasic potassium phosphate are needed? Please walk through this step-by-step.Your goal is to make a buffer with a pH of 4.75 from acetic acid and sodium acetate. Assume the pKa for acetic acid is 4.74. What is the volume of 1 M sodium acetate added to make your buffer? Report your answer to the tenths place. To do this, we use the Henderson-Hasselbach Equation pH = pkA + log (base/acid) where pkA = 4.74 for acetic acid, pH = target pH and [base] /[acid] = x/(1-x) since we don't know the concentration of the base, we will call it x the acid is just 1-x, assuming the two add up to 100% solve for x, and then you know the mL of base and acid to add.Calculate the equivalent weight of Na2CO3 when it is titrated against HCl in presence of phenolphthalein. When a 0.20 M solution of acetic acid is neutralized with 0.20 M NaOH in 0.50 L of water, the pH of the resulting solution will be (given that pKa for CH3COOH = 4.74) If the pKa of formic acid = 3.74, find the pH at the equivalence point in the titration of 25 mL of 0.10 M formic acid with a 0.1 M NaOH solution.
- How the pH at the equivalence point is determined by the species present; why the pH at the midpoint of the buffer region equals the pKa of the acidCan someone please explain why and how the step 1 was done? What was its purpose? Why was only the pKa2 value was used in the second step 2? I don't understand shortcuts and I would appreciate it if it's explained step by step. This was the solution to my previous question in which I asked: What is the pH of a buffer prepared by mixing 100 mL 0.050 mM NaH2PO4 and 25 mL 0.075 mM Na2HPO4? (pKa1=2.2; pKa2= 7.21; pKa3=12.7)Tris or tris(hydroxymethyl)aminomethane is an organic buffering reagent most often used in nucleic acid extractions. Tris (MW = 121.14 g/mol) has a pKa of 8.07 at 25oC, with a white crystalline powder appearance. What is the buffering range/s of Tris buffer? How would you prepare 500mL of a 0.200 M Tris buffer (pH 8.1) solution using Tris and NaTris or sodium Tris (MW = 143.14 g/mol)?
- After a buffer solution of carbonic acid (with pKa of 3.75) was added with 1ml of sulfuric acid, the resulting pH of the carbonic acid buffer solution is 1.25. Do you think the carbonic buffer system still works effectively after the addition of the said acid?a) At temperature 25°C, 0.02 M hydrazine, N2H4 solution is 0.69% ionised. Calculate the i. Concentration of OH- ion ii. ionisation constant, Kb b) Calculate the mass of sodium benzoate, C6H5COONa that should be added to 500 mL of 0.2M aqueous benzoic acid, C6H5COOH solution to produce a buffer with pH 3.50?[ Ka for C6H5COOH = 6.3 x10-5] c) When a 1 x 10-3 moldm-3 solution of CaCl2 is mixed with an equal volume of a 1 x 10-3 moldm-3 solution of Na2SO4, will precipitate form? (Ksp CaSO4= 2 x 10-5) Do you mean that pH? That was all the given information the question gave.. I don't understand how to use the ionised part the mostYou are in charge of making a phosphate buffer at pH 7.5. You are out of solid versions of phosphate,but you find stock solutions of 1.0 M NaH2PO4 and 1.0 M Na2HPO4 in the lab and decide to make thebuffer with these solutions. How much of each of the stock solutions would you mix together to make 200mL of 1.0 M phosphate buffer at pH 7.5? (The pKa values of phosphoric acid are 2.1, 7.2, and 12.3.)

