2. You are measuring the Fe(lI) content of a sample of FeCl2.4H20. You weighed out 1.20g FeCl2.4H20 and dissolved it in 50 ml.s of 0.5M sulfuric acid. You removed 10 ml.s of this solution, mixed it with sulfuric acid and diphenylamine sulfonate indicator, and titrated it to the equivalence point with 0.0167M K2Cr207 solution. You obtained the following data. Volume of dichromate needed to reach the equiv. point = 9.5 ml.s Given that MEeVFe 6MaiVai, (where Mre= molarity of the Fe(lI) solution, VEe is the volume of the Fe(II) solution =10 mls. Mai= molarity of the dichromate solution 0.0167M, and Vdi = volume of dichromat solution = 9.5 ml.s), calculate: %3D %3D %3D (a) The molarity of the Fe(Il) solution ----------M (b) Mass of Fe in the original solution = (MF)(0.05)(55.845) = (c) The theoretical mass % Fe in FeCl2.4H20 -----%
2. You are measuring the Fe(lI) content of a sample of FeCl2.4H20. You weighed out 1.20g FeCl2.4H20 and dissolved it in 50 ml.s of 0.5M sulfuric acid. You removed 10 ml.s of this solution, mixed it with sulfuric acid and diphenylamine sulfonate indicator, and titrated it to the equivalence point with 0.0167M K2Cr207 solution. You obtained the following data. Volume of dichromate needed to reach the equiv. point = 9.5 ml.s Given that MEeVFe 6MaiVai, (where Mre= molarity of the Fe(lI) solution, VEe is the volume of the Fe(II) solution =10 mls. Mai= molarity of the dichromate solution 0.0167M, and Vdi = volume of dichromat solution = 9.5 ml.s), calculate: %3D %3D %3D (a) The molarity of the Fe(Il) solution ----------M (b) Mass of Fe in the original solution = (MF)(0.05)(55.845) = (c) The theoretical mass % Fe in FeCl2.4H20 -----%
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.35QAP
Related questions
Question
100%
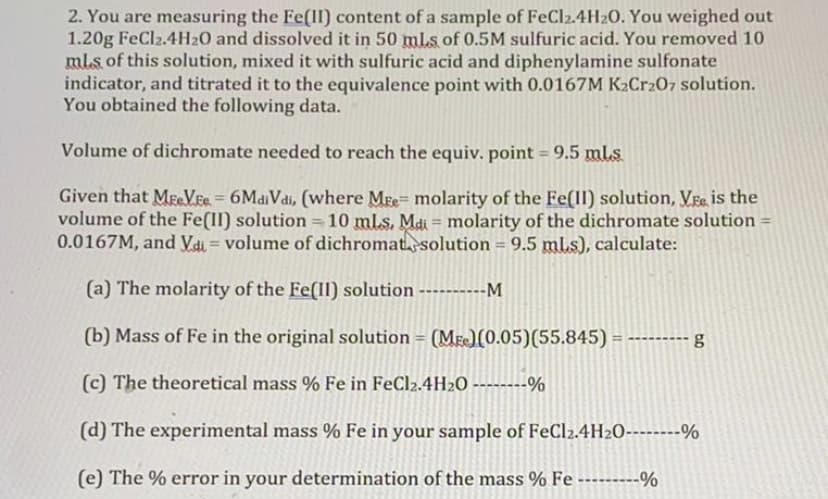
Transcribed Image Text:2. You are measuring the Fe(II) content of a sample of FeCl2.4H20. You weighed out
1.20g FeCl2.4H20 and dissolved it in 50 mls of 0.5M sulfuric acid. You removed 10
ml.s of this solution, mixed it with sulfuric acid and diphenylamine sulfonate
indicator, and titrated it to the equivalence point with 0.0167M K2Cr207 solution.
You obtained the following data.
Volume of dichromate needed to reach the equiv. point = 9.5 ml.s
Given that MeVEe = 6MaiVdi, (where Mre= molarity of the Fe(II) solution, Vre is the
volume of the Fe(II) solution =10 mls. Mai = molarity of the dichromate solution-
0.0167M, and Vai = volume of dichromatsolution = 9.5 ml.s), calculate:
%3D
(a) The molarity of the Fe(Il) solution
-M
(b) Mass of Fe in the original solution = (Me)(0.05)(55.845) = ------
(c) The theoretical mass % Fe in FeCl2.4H20 - -%
(d) The experimental mass % Fe in your sample of FeCl2.4H2O----%
(e) The % error in your determination of the mass % Fe ---------%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning