2.1 ev Group Metastable second 1.67 ev 1.17 ev (1.06 um) 0.50 eV First O ev Ground state Figure 30.65 Neodymium atoms in glass have these energy levels, one of which is metastable. The group of levels above the metastable state is convenient for achieving a population inversion, since photons of many different energies can be absorbed by atoms in the ground state.
2.1 ev Group Metastable second 1.67 ev 1.17 ev (1.06 um) 0.50 eV First O ev Ground state Figure 30.65 Neodymium atoms in glass have these energy levels, one of which is metastable. The group of levels above the metastable state is convenient for achieving a population inversion, since photons of many different energies can be absorbed by atoms in the ground state.
University Physics Volume 3
17th Edition
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:William Moebs, Jeff Sanny
Chapter7: Quantum Mechanics
Section: Chapter Questions
Problem 7.4CYU: Check Your Understanding A sodium atom nukes a transition from the first excited state the wound...
Related questions
Question
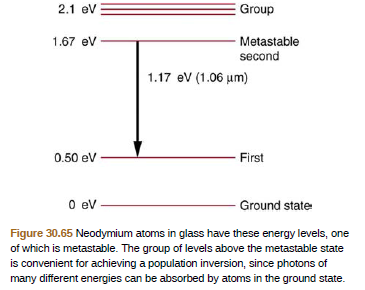
Some of the most powerful lasers are based on the energy levels of neodymium in solids, such as glass, as shown .
(a) What average
1.06 μm
Transcribed Image Text:2.1 ev
Group
Metastable
second
1.67 ev
1.17 ev (1.06 um)
0.50 eV
First
O ev
Ground state
Figure 30.65 Neodymium atoms in glass have these energy levels, one
of which is metastable. The group of levels above the metastable state
is convenient for achieving a population inversion, since photons of
many different energies can be absorbed by atoms in the ground state.
Expert Solution
Answer for part (a)
The energy released during the transition between metastable and the ground state can be given as
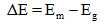
Substituting the values from the energy level diagram gives,
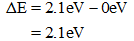
Answer for part (a) continues
Now, the required wavelength can be obtained as
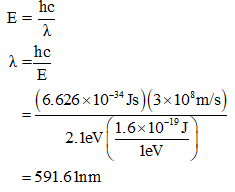
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning