20. What do melting and boiling points (high vs low melting and boiling points) tell us about attractions between molecules? 21. What does the strength of attractions between molecules tell us about the presence of partial charges? 22. Define polarity in terms of strength of attractions and partial charges.
20. What do melting and boiling points (high vs low melting and boiling points) tell us about attractions between molecules? 21. What does the strength of attractions between molecules tell us about the presence of partial charges? 22. Define polarity in terms of strength of attractions and partial charges.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter7: Chemical Bonding And Molecular Structure
Section: Chapter Questions
Problem 7.105PAE
Related questions
Question
I need very short answers they dont have to in complete sentence solve all of them if you cant do all of them dont do anything
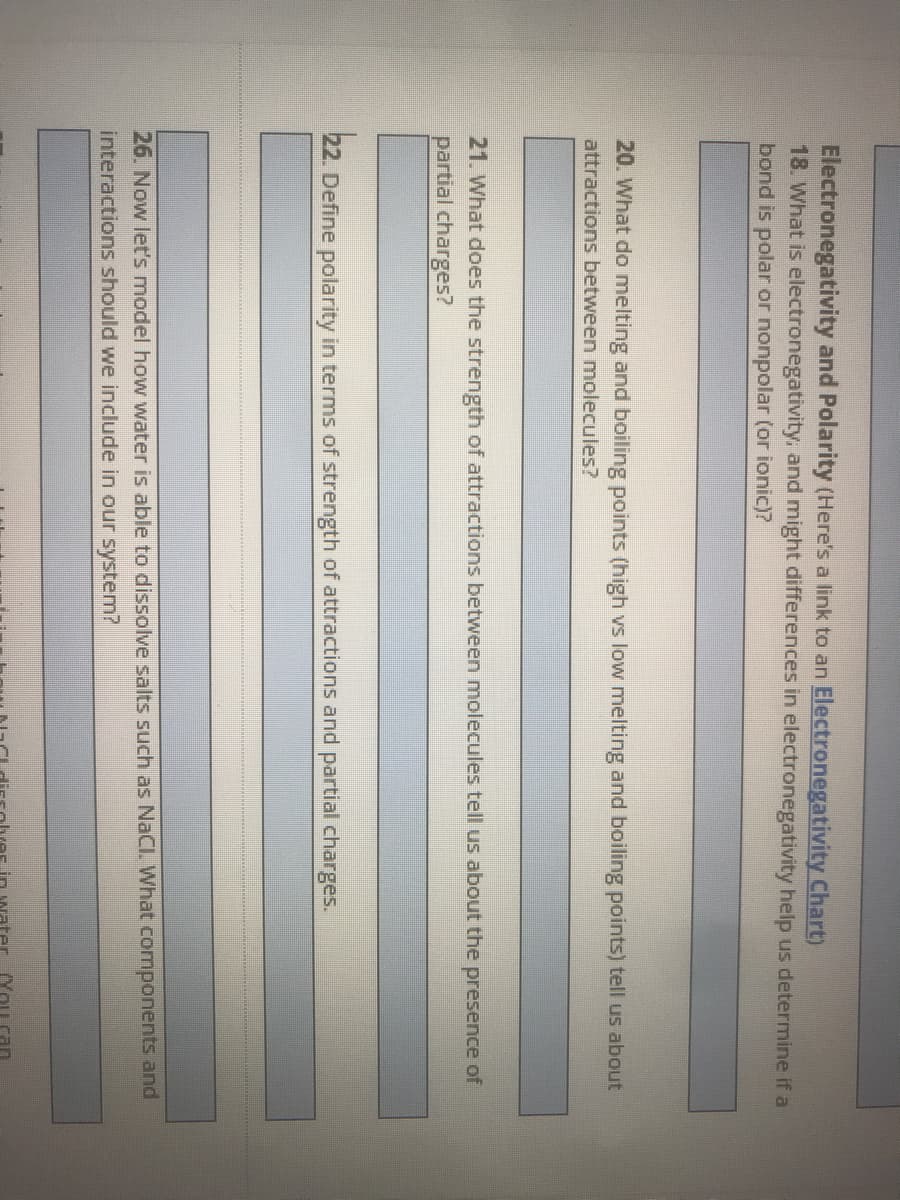
Transcribed Image Text:Electronegativity and Polarity (Here's a link to an Electronegativity Chart)
18. What is electronegativity, and might differences in electronegativity help us determine if a
bond is polar or nonpolar (or ionic)?
20. What do melting and boiling points (high vs low melting and boiling points) tell us about
attractions between molecules?
21. What does the strength of attractions between molecules tell us about the presence of
partial charges?
22. Define polarity in terms of strength of attractions and partial charges.
26. Now let's model how water is able to dissolve salts such as NaCl. What components and
interactions should we include in our system?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning