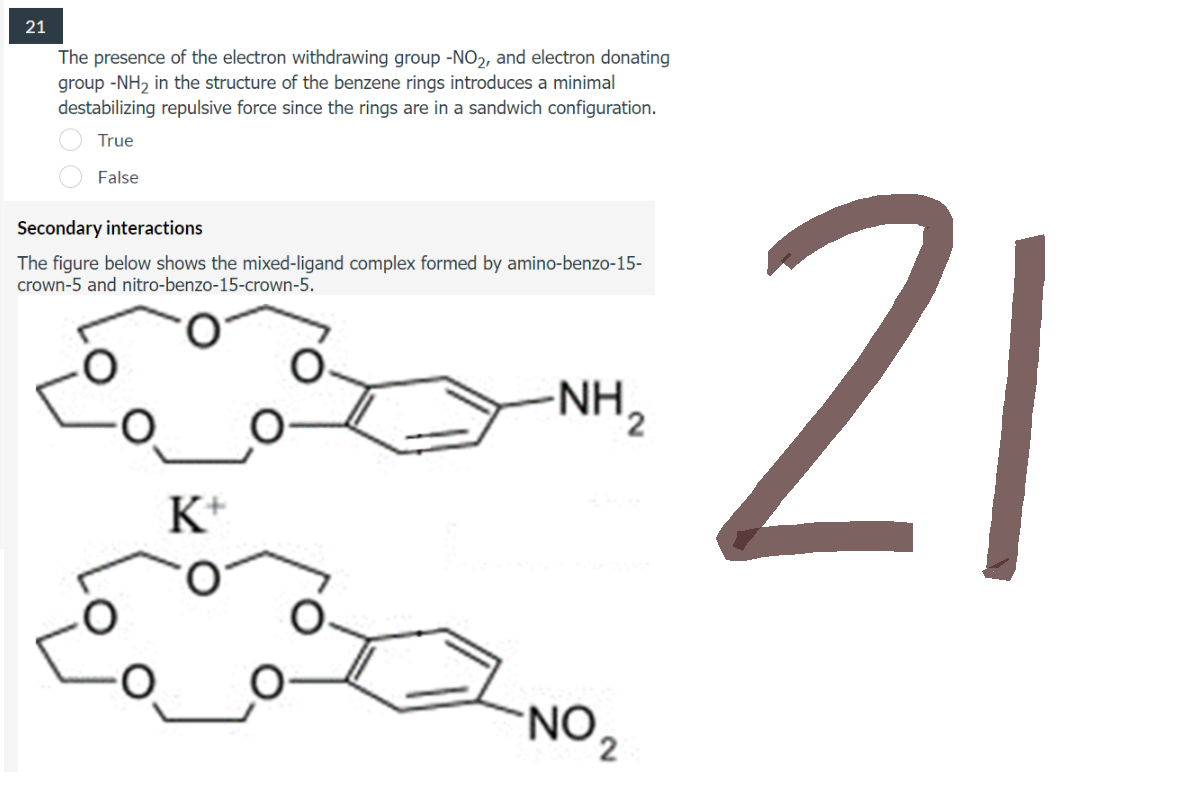
21 The presence of the electron withdrawing group -NO2, and electron donating group -NH₂ in the structure of the benzene rings introduces a minimal destabilizing repulsive force since the rings are in a sandwich configuration. True False Secondary interactions The figure below shows the mixed-ligand complex formed by amino-benzo-15- crown-5 and nitro-benzo-15-crown-5. NH ₂ K+ O NO,
21 The presence of the electron withdrawing group -NO2, and electron donating group -NH₂ in the structure of the benzene rings introduces a minimal destabilizing repulsive force since the rings are in a sandwich configuration. True False Secondary interactions The figure below shows the mixed-ligand complex formed by amino-benzo-15- crown-5 and nitro-benzo-15-crown-5. NH ₂ K+ O NO,
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.14QAP
Related questions
Question
please skip if you already answered this. I will upvote if this is typewritten. much appreciated. thank you so much NOTE: THE BIG NUMBER IS FOR NUMBERING ONLY. IT IS NOT GRADED
Transcribed Image Text:21
The presence of the electron withdrawing group -NO₂, and electron donating
group -NH₂ in the structure of the benzene rings introduces a minimal
destabilizing repulsive force since the rings are in a sandwich configuration.
True
False
Secondary interactions
The figure below shows the mixed-ligand complex formed by amino-benzo-15-
crown-5 and nitro-benzo-15-crown-5.
-NH ₂
K+
NO ₂
21
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning