22-30. (a) The mean free path is the average distance a molecule travels before colliding with another molecule. The mean free path, A, is given by A = kT/(V20P), where k is Boltzmann's constant, T is temperature (K), P is pressure (Pa), and o is the collision cross section. For a molecule with a diameter d, the col- lision cross section is nd. The collision cross section is the area swept out by the molecule within which it will strike any other molecule it encounters. The magnetic sector mass spectrometer is maintained at a pressure of ~10¯³ Pa so that ions do not collide with (and deflect) one another as they travel through the mass analyzer. What is the mean free path of a molecule with a diam- eter of 1 nm at 300 K in the mass analyzer?
22-30. (a) The mean free path is the average distance a molecule travels before colliding with another molecule. The mean free path, A, is given by A = kT/(V20P), where k is Boltzmann's constant, T is temperature (K), P is pressure (Pa), and o is the collision cross section. For a molecule with a diameter d, the col- lision cross section is nd. The collision cross section is the area swept out by the molecule within which it will strike any other molecule it encounters. The magnetic sector mass spectrometer is maintained at a pressure of ~10¯³ Pa so that ions do not collide with (and deflect) one another as they travel through the mass analyzer. What is the mean free path of a molecule with a diam- eter of 1 nm at 300 K in the mass analyzer?
Chapter15: Electrophoresis And Other Separation Methods
Section: Chapter Questions
Problem 5P
Related questions
Question
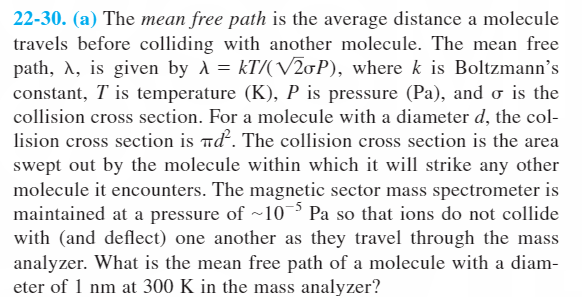
Transcribed Image Text:22-30. (a) The mean free path is the average distance a molecule
travels before colliding with another molecule. The mean free
path, A, is given by A = kT/(V20P), where k is Boltzmann’s
constant, T is temperature (K), P is pressure (Pa), and o is the
collision cross section. For a molecule with a diameter d, the col-
lision cross section is nd. The collision cross section is the area
swept out by the molecule within which it will strike any other
molecule it encounters. The magnetic sector mass spectrometer is
maintained at a pressure of ~103 Pa so that ions do not collide
with (and deflect) one another as they travel through the mass
analyzer. What is the mean free path of a molecule with a diam-
eter of 1 nm at 300 K in the mass analyzer?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning