23. Consider a generic polyprotic acid H2YO3. What is an appropriate combination of acid dissociation constants for this acid? Kal Ka2 A. 2.1 x 104 8.9 x 10-5 В. 3.2 х 10-6 2.2 x 10-10 С. 4.5 х 10-10 6.7 x 10-6 D. 5.3 x 10-5 1.8 x 10-4
23. Consider a generic polyprotic acid H2YO3. What is an appropriate combination of acid dissociation constants for this acid? Kal Ka2 A. 2.1 x 104 8.9 x 10-5 В. 3.2 х 10-6 2.2 x 10-10 С. 4.5 х 10-10 6.7 x 10-6 D. 5.3 x 10-5 1.8 x 10-4
Chapter15: Acid-base Equilibria
Section: Chapter Questions
Problem 23E: Compare the percent dissociation of the acid in Exercise 21a with the percent dissociation of the...
Related questions
Question
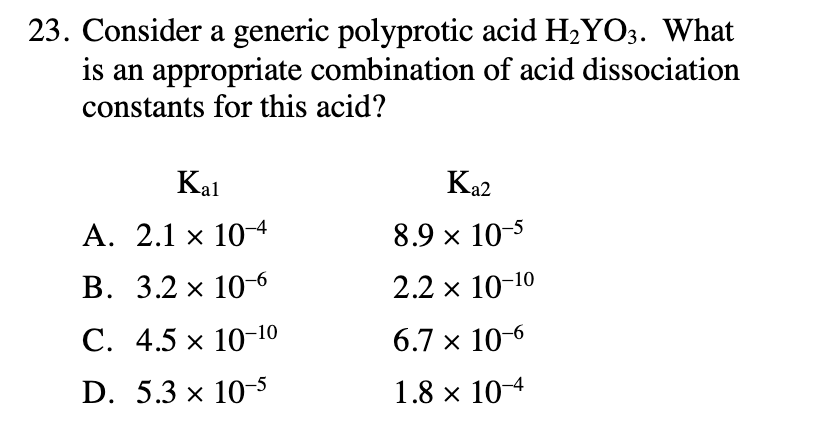
Transcribed Image Text:23. Consider a generic polyprotic acid H2YO3. What
is an appropriate combination of acid dissociation
constants for this acid?
Kal
Ka2
A. 2.1 x 104
8.9 x 10-5
В. 3.2 х 10-6
2.2 x 10-10
С. 4.5 х 10-10
6.7 x 10-6
D. 5.3 x 10-5
1.8 x 10-4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning