25. (a) What is a Brønsted-Lowry acid? (b) What is a Brønsted-Lowry base? (c) Use the following chemical equation to explain these concepts and (d) to identify the conjugate acid-base pairs. Point form is both accepted and expected for this question. NH3(ag) + HCN = NH4 (aq) + CN" (aq) (aq)
25. (a) What is a Brønsted-Lowry acid? (b) What is a Brønsted-Lowry base? (c) Use the following chemical equation to explain these concepts and (d) to identify the conjugate acid-base pairs. Point form is both accepted and expected for this question. NH3(ag) + HCN = NH4 (aq) + CN" (aq) (aq)
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter4: Acids And Bases
Section: Chapter Questions
Problem 4.35AP: The sec-butyl cation can react as both a Brnsted-Lowry acid (a proton donor) and a Lewis acid (an...
Related questions
Question
100%
Pls help ASAP.
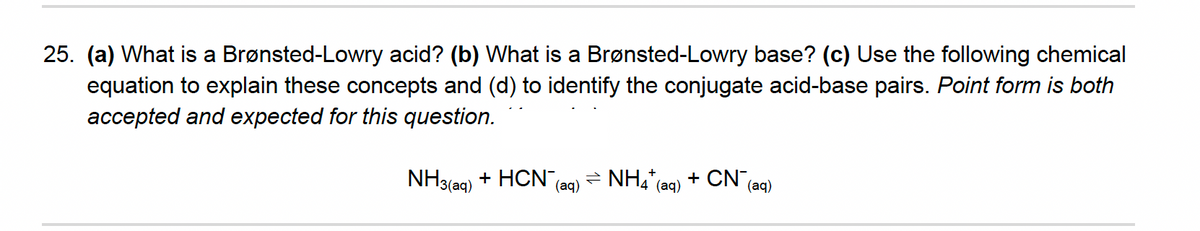
Transcribed Image Text:25. (a) What is a Brønsted-Lowry acid? (b) What is a Brønsted-Lowry base? (c) Use the following chemical
equation to explain these concepts and (d) to identify the conjugate acid-base pairs. Point form is both
accepted and expected for this question.
NH3(aq)
+ HCN,
- NH4"(ag) + CN
(aq)
(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
