27) Which region of the following phase diagram represents solid? Which curve represents the vaporization-condensation process? b. B. A Temperature A) b, BD В) а, BD C) c, CD D) d, AB E) а, СВ 28) Classify each substance in the table as either a metallic, ionic, molecular, or covalent network solid: Substance Electrical Conductivity only if melted/dissolved high Melting Point Appearance brittle, white shiny, malleable 1100 °C hard, colorless Solubility in Water soluble 800 °C Y insoluble 3550 °C none insoluble A) X: ionic; Y: covalent network; Z: metallic B) X: ionic; Y: metallic; Z: molecular C) X: ionic; Y: metallic; Z: covalent network 29) Identify the type of crystalline solid (metallic, network covalent, ionic, or molecular) formed by C (diamond): A) ionic B) metallic Ccovalent network D) molecular 30) Identify the type of crystalline solid (metallic, network covalent, ionic, or molecular) formed by C,H,0H: A molecular B) covalent network C) ionic D) metallic Pressure
27) Which region of the following phase diagram represents solid? Which curve represents the vaporization-condensation process? b. B. A Temperature A) b, BD В) а, BD C) c, CD D) d, AB E) а, СВ 28) Classify each substance in the table as either a metallic, ionic, molecular, or covalent network solid: Substance Electrical Conductivity only if melted/dissolved high Melting Point Appearance brittle, white shiny, malleable 1100 °C hard, colorless Solubility in Water soluble 800 °C Y insoluble 3550 °C none insoluble A) X: ionic; Y: covalent network; Z: metallic B) X: ionic; Y: metallic; Z: molecular C) X: ionic; Y: metallic; Z: covalent network 29) Identify the type of crystalline solid (metallic, network covalent, ionic, or molecular) formed by C (diamond): A) ionic B) metallic Ccovalent network D) molecular 30) Identify the type of crystalline solid (metallic, network covalent, ionic, or molecular) formed by C,H,0H: A molecular B) covalent network C) ionic D) metallic Pressure
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 66QAP
Related questions
Question
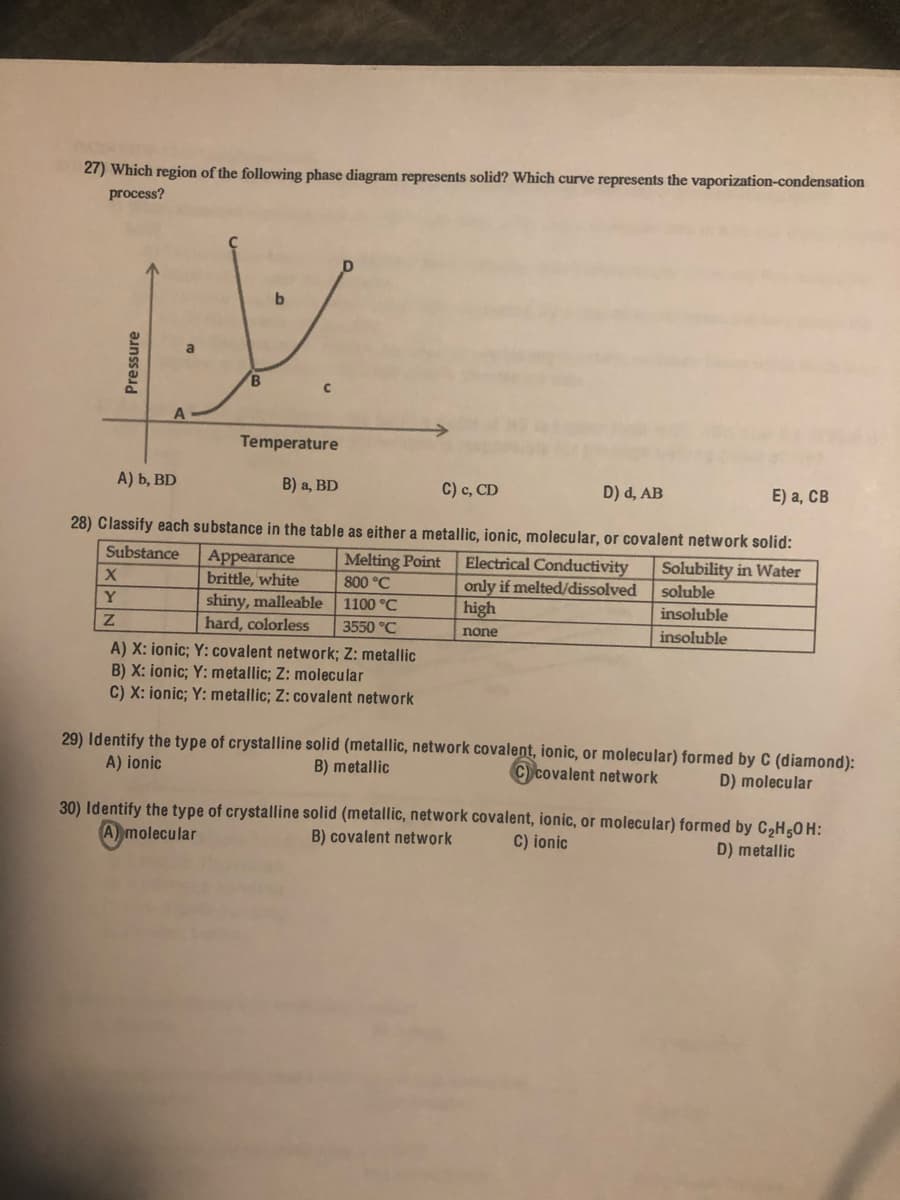
Transcribed Image Text:27) Which region of the following phase diagram represents solid? Which curve represents the vaporization-condensation
process?
B.
Temperature
A) b, BD
B) a, BD
C) c, CD
D) d, AB
E) а, СВ
28) Classify each substance in the table as either a metallic, ionic, molecular, or covalent network solid:
Substance
Appearance
brittle, white
shiny, malleable
hard, colorless
A) X: ionic; Y: covalent network; Z: metallic
B) X: ionic; Y: metallic; Z: molecular
C) X: ionic; Y: metallic; Z: covalent network
Melting Point
800 °C
Electrical Conductivity
only if melted/dissolved
high
Solubility in Water
soluble
Y
1100 °C
insoluble
3550 °C
none
insoluble
29) Identify the type of crystalline solid (metallic, network covalent, ionic, or molecular) formed by C (diamond):
A) ionic
B) metallic
Ccovalent network
D) molecular
30) Identify the type of crystalline solid (metallic, network covalent, ionic, or molecular) formed by C2H50 H:
A) molecular
B) covalent network
C) ionic
D) metallic
Pressure
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning