A student prepared the graph shown based on the vapor 10 pressure of a liquid over a range of temperatures. The best fit line was found to have the equation 9. 8. y = -1978x + 12.4 y = -1978x + 12.4 7- Use this data to calculate the heat of vaporization of the liquid. 3- heat of vaporization = kJ/mol 2- 0+ 0.002 0.003 0.004 0.005 0.006 I/T (K) In P (mmHg)
A student prepared the graph shown based on the vapor 10 pressure of a liquid over a range of temperatures. The best fit line was found to have the equation 9. 8. y = -1978x + 12.4 y = -1978x + 12.4 7- Use this data to calculate the heat of vaporization of the liquid. 3- heat of vaporization = kJ/mol 2- 0+ 0.002 0.003 0.004 0.005 0.006 I/T (K) In P (mmHg)
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.73E: Why are steam burns so much worse than water burns even if the H2O is at the same temperature for...
Related questions
Question
I need full detail and accurate answer.
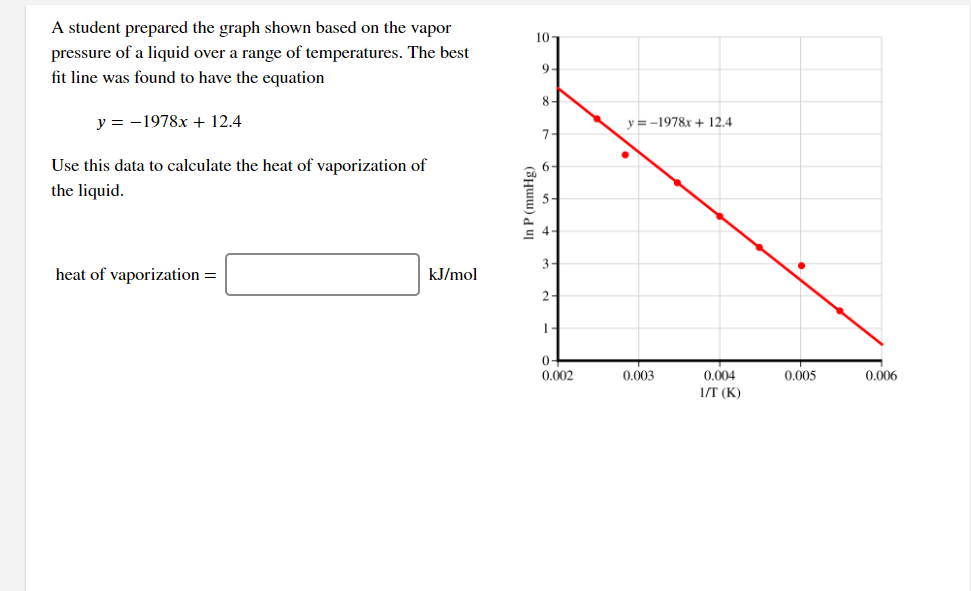
Transcribed Image Text:A student prepared the graph shown based on the vapor
10
pressure of a liquid over a range of temperatures. The best
fit line was found to have the equation
9-
8-
y = -1978x + 12.4
y =-1978x + 12.4
7.
Use this data to calculate the heat of vaporization of
the liquid.
6.
3
heat of vaporization =
kJ/mol
2-
1
0.002
0.003
0.004
0.005
0.006
1/T (K)
(3Hwu) du
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning