27. According to the picture below, which statement is false? Visible Light 700nm 600nm 500nm 400nm Radio waves Microwaves Infrared Ultraviolet X-rays Gamma -LONGER WAVELENGTH (meters) SHORTER- 102 1 1 101 102 10 104 10s 10 107 10 10° 1010 1011 1012 1013 (A) Ultraviolet rays have a higher frequency than microwaves. (B) As wavelength gets shorter, the energy increases. (C) Red light (wavelength = 700nm) has less energy than blue light (wavelength = 500nm). (D) Radio waves have the shortest wavelength.
27. According to the picture below, which statement is false? Visible Light 700nm 600nm 500nm 400nm Radio waves Microwaves Infrared Ultraviolet X-rays Gamma -LONGER WAVELENGTH (meters) SHORTER- 102 1 1 101 102 10 104 10s 10 107 10 10° 1010 1011 1012 1013 (A) Ultraviolet rays have a higher frequency than microwaves. (B) As wavelength gets shorter, the energy increases. (C) Red light (wavelength = 700nm) has less energy than blue light (wavelength = 500nm). (D) Radio waves have the shortest wavelength.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter6: The Structure Of Atoms
Section6.3: Atomic Line Spectra And Niels Bohr
Problem 6.4CYU: The Lyman series of spectral lines for the H atom, in the ultraviolet region, arises from...
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
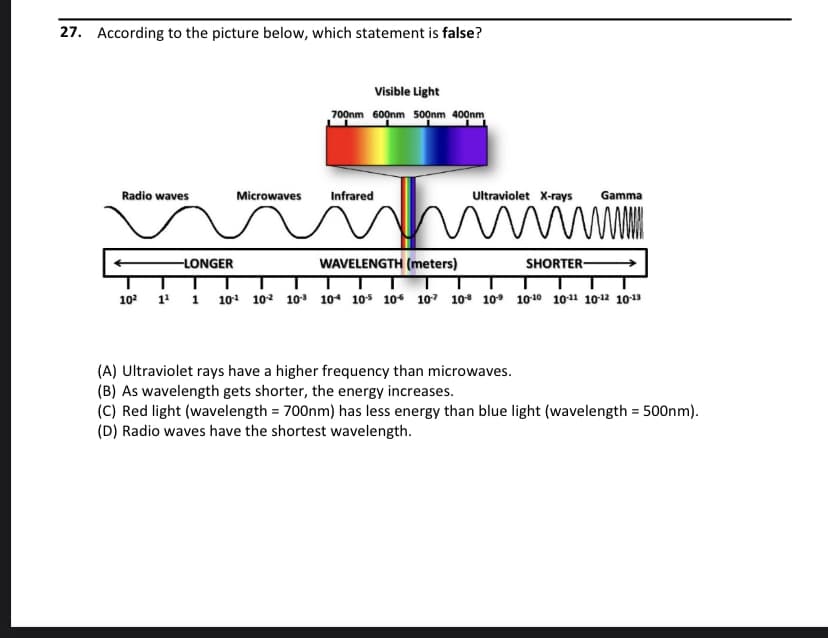
Transcribed Image Text:27. According to the picture below, which statement is false?
Visible Light
700nm 600nm 50nm 400nm
Radio waves
Microwaves
Infrared
Ultraviolet X-rays
Gamma
-LONGER
WAVELENGTH (meters)
SHORTER-
102
1 1 101 10² 10 104 10s 10 107 10* 10° 1010 1011 1012 1013
(A) Ultraviolet rays have a higher frequency than microwaves.
(B) As wavelength gets shorter, the energy increases.
(C) Red light (wavelength = 700nm) has less energy than blue light (wavelength = 500nm).
(D) Radio waves have the shortest wavelength.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning