2Ca(s) +02(g)- 2Ca0(s) AH =-1269 8 kJ; AS =-364.6 J/K Part A For this problem, assume that all reactants and products are in their standard states. Calculate the free energy change for the reaction at 35°C. You may want to reference (Pages 811 815) Section 18.6 while completing this problem. Express your answer using four significant figures. AG= %3D
2Ca(s) +02(g)- 2Ca0(s) AH =-1269 8 kJ; AS =-364.6 J/K Part A For this problem, assume that all reactants and products are in their standard states. Calculate the free energy change for the reaction at 35°C. You may want to reference (Pages 811 815) Section 18.6 while completing this problem. Express your answer using four significant figures. AG= %3D
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter16: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 21Q
Related questions
Question
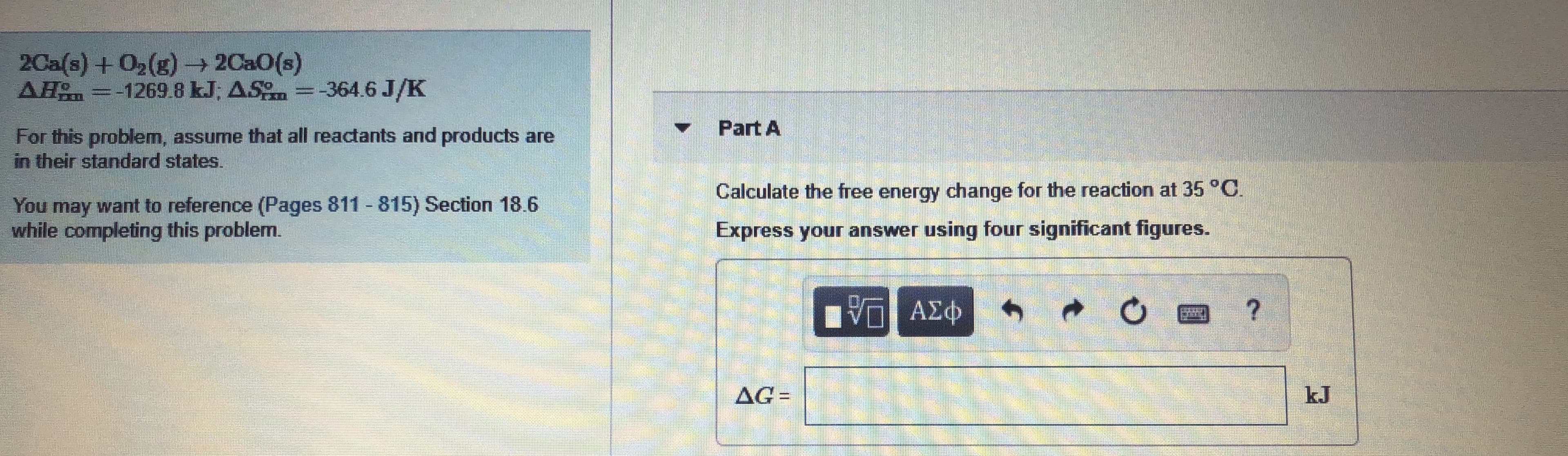
Transcribed Image Text:2Ca(s) +02(g)- 2Ca0(s)
AH =-1269 8 kJ; AS =-364.6 J/K
Part A
For this problem, assume that all reactants and products are
in their standard states.
Calculate the free energy change for the reaction at 35°C.
You may want to reference (Pages 811 815) Section 18.6
while completing this problem.
Express your answer using four significant figures.
AG=
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning