2H2O(g) Reaction 2 heat BaCl, 2H2O(s) BaCl2e) + BaO(s) H2O(9) 2HCI (9) Reaction 3 heat + + BaCl, 2H2O(s) QUESTION If the mass of BaCl, 2H,0s) sample subject to thermal decomposition was 2.526 g, what is the theoretical yield of BaCl(s) if Reaction 2 occurred. (The molar mass of BaCl, 2H2O6, is 244.2 g; the molar mass of BaCls) is 208.2 g) 2(s)
2H2O(g) Reaction 2 heat BaCl, 2H2O(s) BaCl2e) + BaO(s) H2O(9) 2HCI (9) Reaction 3 heat + + BaCl, 2H2O(s) QUESTION If the mass of BaCl, 2H,0s) sample subject to thermal decomposition was 2.526 g, what is the theoretical yield of BaCl(s) if Reaction 2 occurred. (The molar mass of BaCl, 2H2O6, is 244.2 g; the molar mass of BaCls) is 208.2 g) 2(s)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section: Chapter Questions
Problem 119QRT
Related questions
Question
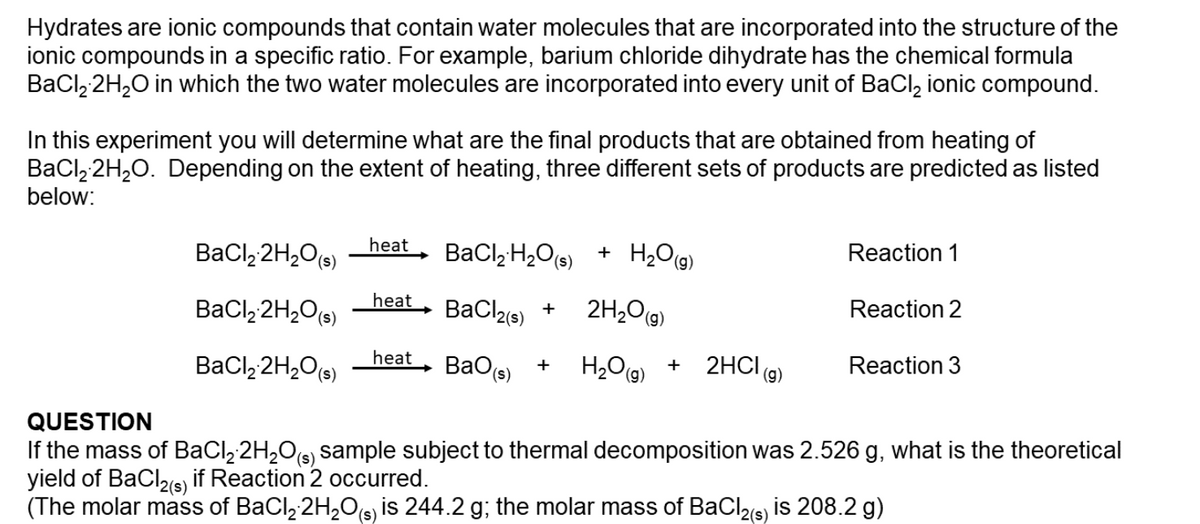
Transcribed Image Text:Hydrates are ionic compounds that contain water molecules that are incorporated into the structure of the
ionic compounds in a specific ratio. For example, barium chloride dihydrate has the chemical formula
BaCl, 2H20 in which the two water molecules are incorporated into every unit of BaCl, ionic compound.
In this experiment you will determine what are the final products that are obtained from heating of
BaCl, 2H,0. Depending on the extent of heating, three different sets of products are predicted as listed
below:
+ H2O(g)
Reaction 1
BaCl, 2H,O(s)
heat
BaCl,H,O(s)
2H20(9)
Reaction 2
heat
BaCl, 2H,O(s)
BaClae) +
heat
BaO(s)
H2Og)
2HCI (9)
Reaction 3
+
BaCl, 2H,O(s)
QUESTION
If the mass of BaCl, 2H,O(s) sample subject to thermal decomposition was 2.526 g, what is the theoretical
yield of BaClis) if Reaction 2 occurred.
(The molar mass of BaCl, 2H,Os, is 244.2 g; the molar mass of BaCl2(s) is 208.2 g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning