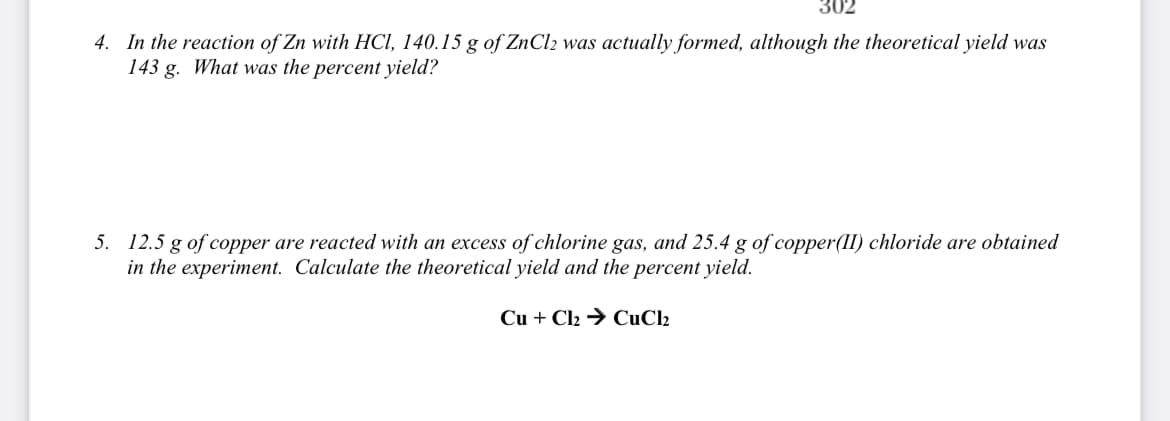
4. In the reaction of Zn with HCI, 140.15 g of ZNCI2 was actually formed, although the theoretical yield was 143 g. What was the percent yield? 5. 12.5 g of copper are reacted with an excess of chlorine gas, and 25.4 g of copper(II) chloride are obtained in the experiment. Calculate the theoretical yield and the percent yield. Cu + Cl2 → CuCl2
4. In the reaction of Zn with HCI, 140.15 g of ZNCI2 was actually formed, although the theoretical yield was 143 g. What was the percent yield? 5. 12.5 g of copper are reacted with an excess of chlorine gas, and 25.4 g of copper(II) chloride are obtained in the experiment. Calculate the theoretical yield and the percent yield. Cu + Cl2 → CuCl2
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 70QRT
Related questions
Question
Transcribed Image Text:302
4. In the reaction of Zn with HCI, 140.15 g of ZNCI2 was actually formed, although the theoretical yield was
143 g. What was the percent yield?
5. 12.5 g of copper are reacted with an excess of chlorine gas, and 25.4 g of copper(II) chloride are obtained
in the experiment. Calculate the theoretical yield and the percent yield.
Cu + Cl2 → CuCl2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning