3-1. For each of the given amino acids, write the equilib- rium reactions that would apply at a pH corresponding to each of the pK, values for each amino acid: (a) alanine; (b) glutamic acid; (c) arginine; (d) a₁, e-diaminopimelic acid (pK.. = 1.8, pK₁₂ = 2.2, pK, = 8.8, pK,,. = 9.9), whose for- mula is a4
Q: Which of the following exhibit(s) the "quaternary" structure of nucleic acids? a.ribosome…
A: Quaternary structure of nucleic acids refers to the higher-level organization of nucleic acids,…
Q: Draw the structure of Asparagine and identify all H-Bond donors and acceptors
A: “Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Monosaccharides should always have at least two hydroxyl group. True or False? Monosaccharides can…
A: Note : Hi ! Thank you for the question. We are authorized to answer three subparts at a time. Since…
Q: Carbamoyl phosphate is formed from a.ATP b.C02 c.H20 d.NADH
A: The urea cycle is the biochemical pathway that converts ammonia into urea. The pathway channels all…
Q: What are the charges of the following amino acids peptides at ph 14? 1. GLAVV 2. RRKKQ
A: Peptide chain is a short chain of amino acids joined by peptide/amide bonds (covalent bond). The…
Q: list the amino acids that will carry a net charge at pH 7 within a protein. What is the charge at pH…
A: Note : Hi ! Thank you for the question. We are authorized to answer one question at a time. Since…
Q: Structures of L-Cysteine from highly protonated to depronated form. Note Structure A as the most…
A: Proteins are unbranched polymers constructed from 20 standard α-amino acids. They have four levels…
Q: Which of the following statements is/are TRUE for DNA Replication? Two daughter DNAs are formed,…
A: DNA replication is a process by which 2 copies of DNA are produced from a DNA double helix using…
Q: 6) Proline is not commonly found in the middle of a-helices because its ring structure prevents it…
A: A protein's function depends on its structure. There are four levels of protein structure: primary,…
Q: 3.-Substances are removed from the cell-due-to-the- connection of the membrane structure of the…
A: INTRODUCTION Endocytosis is the process of capturing a substance or particle from outside the cell…
Q: The amino acid histidine has ionizable groups with p?a values of 1.8, 6.0, and 9.2, as shown. A…
A: Amino acids are the building blocks of proteins which are composed of amino group (NH3+), carboxyl…
Q: 1. Why are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) important?
A: Since you have asked multiple questions we will solve the first question for you. If you want any…
Q: Which of the following processes is described in the reaction shown in the picture?…
A: The enzyme glutamate dehydrogenase catalyzes a reaction in which ammonium ion directly combines with…
Q: Which proteins help other polypeptides to fold into the right shape?
A: Proteins are composed of amino acids. They are linked together by peptide linkages. Proteins have…
Q: Q3.2- In the Anfinsen protein folding experiment, removing urea and BME at the same time led to…
A: Anfinsen protein folding - is based on the thermodynamic protein folding hypothesis. It explain…
Q: Enzyme Urease Chymotrypsin Alcohol Dehydrogenase Lysozyme Enzyme Official Name (write N/A if…
A: Urease- an enzyme produced by many bacteria, fungi etc. It hydrolyzes urea into ammonia and carbon…
Q: The Na+/K+ ATPase pump is a P-type pump and requires a covalent phosphorylation from a phosphate…
A: ATPase is the most common example of primary active transport. Virtually every animal cell…
Q: 2. What is the secondary structure of protein? A. The oligomer protein B. Local conformation of the…
A: Proteins have four conformational levels known as primary, secondary, tertiary, and quaternary. All…
Q: ELISA link____ to___ in order to create a colorimetric assay
A: Antigens and antibodies react specifically to form the Antigen-Antibody complex. Based on this…
Q: Galactosemia is due to? A. lactase deficiency B. accumulation of lactic acid C. accumulation…
A: Galactosemia is a metabolic disorder, due to the deficiency of the enzyme galactose-1-phosphate…
Q: is the active component in Benedict's Reagent is the storage from of carbohydrates in plants is the…
A: These tests are used to detect the presence of carbohydrates or reducing sugars or the presence of…
Q: 8. The physical chemical properties of proteins and their colloidal solutions. Precipitation of…
A: Protein is a type of biological macromolecule. These are high molecular weight nitrogen-rich…
Q: Identify if the statements are TRUE or FALSE. If false, write the word/s that make(s) the statement…
A: Transamination is a chemical reaction that is responsible for the deamination of most amino acids.…
Q: The enzyme β-methylaspartase catalyzes the deamination of β-methylaspartate. For this aspartate…
A: Enzymes are protein molecules that increase the rate of reaction by decreasing the activation…
Q: 9.3 Identify which of the following are a-amino acids. CH3 | H₂N-C-COOH CH, a. b. H H₂N-C-CH₂-COOH…
A: The proteins are constituted of 20 naturally occurring amino acids. The amino acids are all alpha…
Q: What would the primary ATP production pathway(s) and fuel source(s) be for a competitive basketball…
A: Skeletal muscle can use free fatty acids, ketone bodies, or glucose as fuel, depending on the degree…
Q: Which of the following statements is/are TRUE for transcription? A. RNA polymerase uses one strand…
A: Transcription is the process in which RNA is synthesized from DNA. This process involves certain…
Q: Which of the following statements regarding hydrogen bonding in secondary structures is true?…
A: A weak chemical link that exists between a hydrogen atom that is partly positively charged and an…
Q: 9. A patient with liver failure underwent a study of the electrophoretic spectrum of serum proteins.…
A: Proteins are the complex molecules that are made up of Aminoacids. They have definite charge that is…
Q: 2.) An unknown amino acid was subjected to paper chromatography with five amino acid standards in…
A: Paper chromatography is a biochemical technique that is used to separate a mixture of substances…
Q: 4. Describe aldonic acids, uronic acids, alditols, deoxy sugars, and amino sugars.
A: Modified monosaccharides are called sugar derivatives. Monosaccharide molecules that have undergone…
Q: Which of the following statements does NOT apply to Mg2+ It is important in the mammalian aldolase…
A: Mammalian aldolase (Class I aldolase) catalyzes the aldol condensation reaction where fructose…
Q: a. Synthesizing glutamine is endergonic and so not spontaneous. How would having hese two reactions…
A: Coupled bioenergetics reactions are set of chemical reaction in which energy is moved from one side…
Q: 7. The surgeon used a 70% solution of ethyl alcohol to treat the hands before surgery. What is the…
A: 70% ethyl alcohol is used to sanitize the hands and hence, is used by the surgeon before the…
Q: Which of the following membranes would be MOST fluid? One containing lipids with saturated 16…
A: Membrane fluidity gets increased when there is a high proportion of unsaturated fatty acids that…
Q: In the chain elongation of proteins, the new aminoacyl-tRNA bonds to? a.A site b.E site c.G site…
A: Translation is process by which the cells produce protein from the information provided by the DNA.…
Q: Which of the following are nitrogenous bases with the general structure? a.guanine b.uracil…
A:
Q: Type of DNA spontaneous mutation that can explain why uracil is not found in DNA a.depurination…
A: DNA is composed of adenine, thymine, cytosine and guanine.Uracil is not found in the structure of…
Q: 10. Explain the difference at the molecular level in how temperature and urea denature a protein.
A: The three dimensional structure of a protein is held together by noncovalent interactions like…
Q: Write TRUE or FALSE. If false, write the word/s that make(s) the statement incorrect. 1.Ile and…
A: Proteins are made up of amino acids and these amino acids are linked via peptide bond. The amino…
Q: What is the net charge on the peptide RHTLE at pH 12.5?
A: Net charge of a peptide is calculated by adding the charges of individual amino acids in the…
Q: Which of the following statements is/are TRUE? A. Cells in a resting metabolic state have low ATP…
A: Introduction: Lipid is one of the important sources of energy required for our body. It primarily…
Q: Aerobic respiration takes place in three stages: transition, Krebs cycle, and electron transport.…
A: Areobic Respiration : The process by which cells break down Glucose and generate ATP or energy. The…
Q: In an antibody, the heavy chain polypeptides are attached to the light chain polypeptides through…
A: Since antibody is a tetrameric structure consisting of 2 light chains and heavy chains so it is a…
Q: 8. Predict the effect of each of the following environmental changes on the pKa of a glutamate side…
A: The proteins are made of twenty naturally occurring amino acids. The net charge on the alpha ,…
Q: Which statement best describes the definition of the term amphoteric? 1.Amino acids are soluble in…
A: Amino acids are organic molecules that act as the component units of proteins. Acids are proton…
Q: For one molecule of the fatty acid as shown in the picture. How many molecules of NADH can be…
A: During the absence of carbohydrates, fatty acids are degraded to meet the energy demands of the…
Q: Which of the following is CORRECT about the movement of e- in photosynthesis? A. H20 → Pheo → PQ →…
A: Photosynthesis is the process by which plants utilise sunlight to generate energy required for their…
Q: What is the significance of acetyl-CoA to lipid metabolism?
A: The synthesis and breakdown of lipids in cells is known as lipid metabolism. It involves the storing…
Part D only
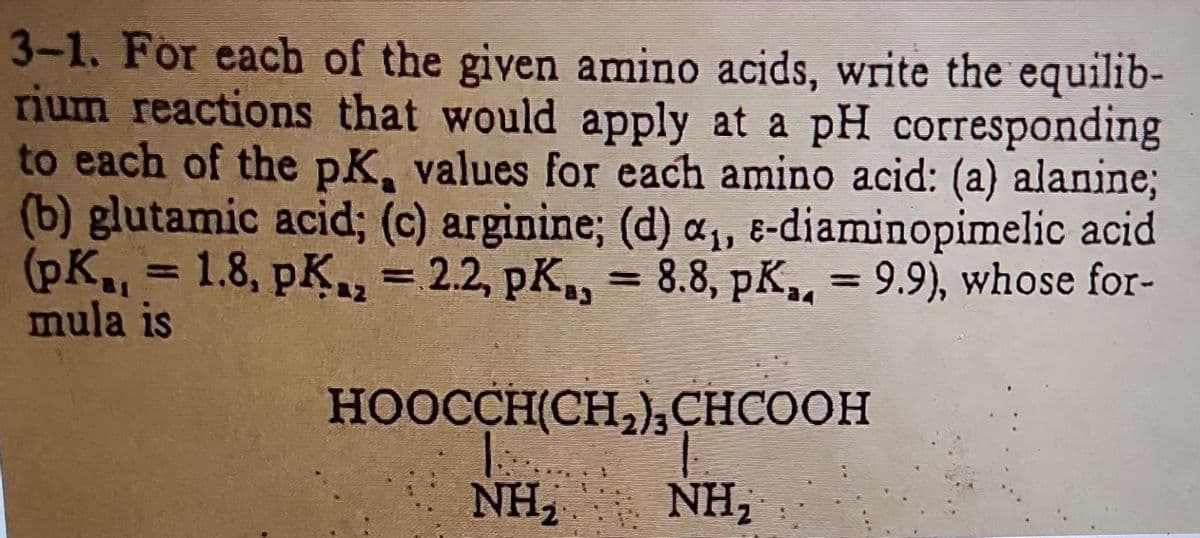
Step by step
Solved in 2 steps with 2 images

- 1. In a protein, why does when Ala is replaced with Ile, it loses its activity but when Lys is replaced by Arg and Leu to Ile, it only has little effect on protein structure and function? Explain. 2. Why do proteins cannot be denatured reversibly when they are chemically altered to change the chemical composition of certain side chains? Explain.6 (a) A decapeptide has the following amino acid composition: Ala2 , Arg, Cys, Glu, Gly, Leu, Lys, Phe, Val Partial hydrolysis yields the following tripeptides: Cys-Glu-Leu + Gly-Arg-Cys + Leu-Ala-Ala+ Lys-Val-Phe + Val-Phe-Gly. Reaction of the decapeptide with 2,4-dinitrofluorobenzene yields 2,4-dinitrophenylysine. From the experimental data, deduce the primary structure of the decapeptide. (b) Suggest a scheme you will follow to synthesize the dipeptide Ala-Gly1. Draw the tetrapeptide Met-Ala-Thr-Thr at a ph of 7? 2. Draw the tetrapeptide Met-Ala-Thr-Thr at a ph of 12?
- 1. A certain polypeptide was treated with trypsin and yielded the following Fragments: Leu-Glu Gly-Tyr-Asn-Arg Gln-Ala-Phe-Val-Lys The same polypeptide was treated with chymotrypsin and yielded the following fragments: Gln-Ala-Phe Asn-Arg-Leu-Glu Val-Lys-Gly-Tyr What is the amino acid sequence of this polypeptide? Instructions Make use of the table below to determine the sequence of the mystery protein.Why is it impossible for humans to digest food that contains cellulose?Describe the differences in the four protein structures.
- Amino acids have the generic structure seen below, where R represents different carbon-based side chains. Describe how the structure of amino acids allows them to be linked into long peptide chains to form proteins.1. Sickle cell anemia results from a substitution of a valine for a glutamic acid. What do you expect the effect might be if the mutation were to have placed a leucine at that site? An aspartic acid? 2. Of the following amino acids, glycine, isoleucine, and lysine, which would you expect to be the most soluble in an acidic aqueous solution? Which the least? 3. How many structural isomers could be formed from a molecule with the formula C5H12? C4H8?1. At pH 7, draw the structure of Arg-Tyr-Gln-Glu-Lys. 2. What’s the charge of this peptide at pH 12? Explain.
- 1.)At what pH are each of the amino acids present as zwitterions, and was there any point with any beakers where the pH was not changing even though you were adding NaOH or HCI?using, 3’ TGAGGCGCTAGGCCAAGCGGTAAGGATGCATGGTCGTGGTAG , What would be the resultant type of error on the amino acid chain?15. The free energy of folding of a protein is -17kJ/mol. What tenoerature (C) do you have to heat the protein to unfold 10% of the proteins in solution?

