The amino acid histidine has ionizable groups with p?a values of 1.8, 6.0, and 9.2, as shown. A biochemist makes up 95 mL of a 0.15 M solution of histidine at a pH of 5.3. She then adds 60 mL of 0.10 M HCl. What is the pH of the resulting solution?
The amino acid histidine has ionizable groups with p?a values of 1.8, 6.0, and 9.2, as shown. A biochemist makes up 95 mL of a 0.15 M solution of histidine at a pH of 5.3. She then adds 60 mL of 0.10 M HCl. What is the pH of the resulting solution?
Chapter1: Introduction To The Structural Units
Section: Chapter Questions
Problem 3FIB
Related questions
Question
The amino acid histidine has ionizable groups with p?a values of 1.8, 6.0, and 9.2, as shown.
A biochemist makes up 95 mL of a 0.15 M solution of histidine at a pH of 5.3. She then adds 60 mL of 0.10 M HCl. What is the pH of the resulting solution?
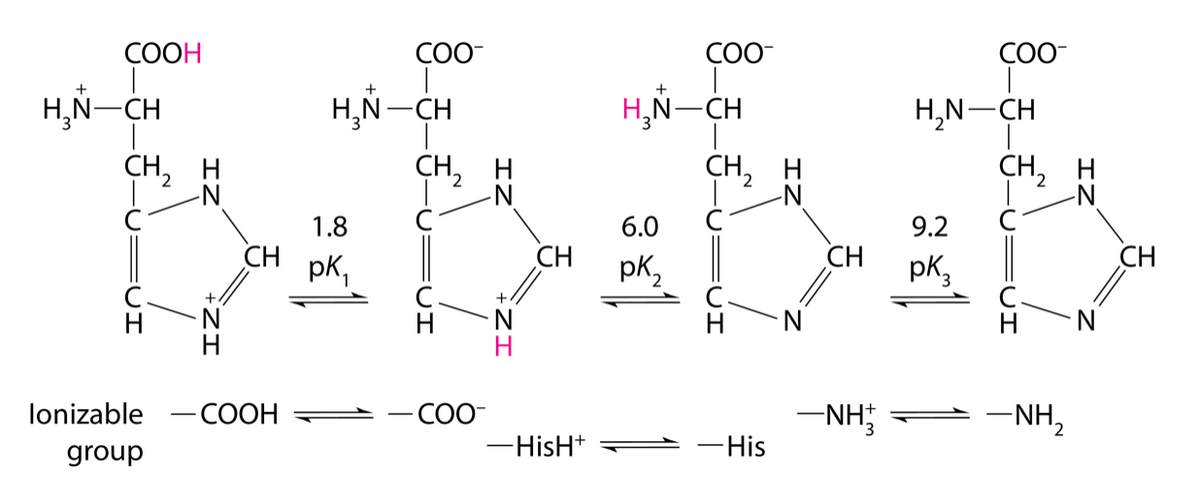
Transcribed Image Text:COOH
H₂N-CH
CH ₂
H
CH
lonizable -COOH
group
COO-
H₂N-CH
T
1.8
pk₁
CH2
COO-
H
CH
-HisH+
COO™
H₂N-CH
6.0
pk₂
CH, H
-His
CH
-NH
COO™
H₂N-CH
I
CH₂ H
9.2
pk3
-NH₂
CH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you








Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage
