3 2 4 Reaction Coordinate 7. Is this reaction endothermic or exothermic? exotherm TC 8. Identify the arrow that represents: a. The activation energy of the forward reaction b. The potential energy of the activated complex c. The potential energy of the reactants d. The difference in energy between reactants and products (AH) Potential Energy
3 2 4 Reaction Coordinate 7. Is this reaction endothermic or exothermic? exotherm TC 8. Identify the arrow that represents: a. The activation energy of the forward reaction b. The potential energy of the activated complex c. The potential energy of the reactants d. The difference in energy between reactants and products (AH) Potential Energy
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 114AP: . What does the activation energy for a reaction represent? How is the activation energy related to...
Related questions
Question
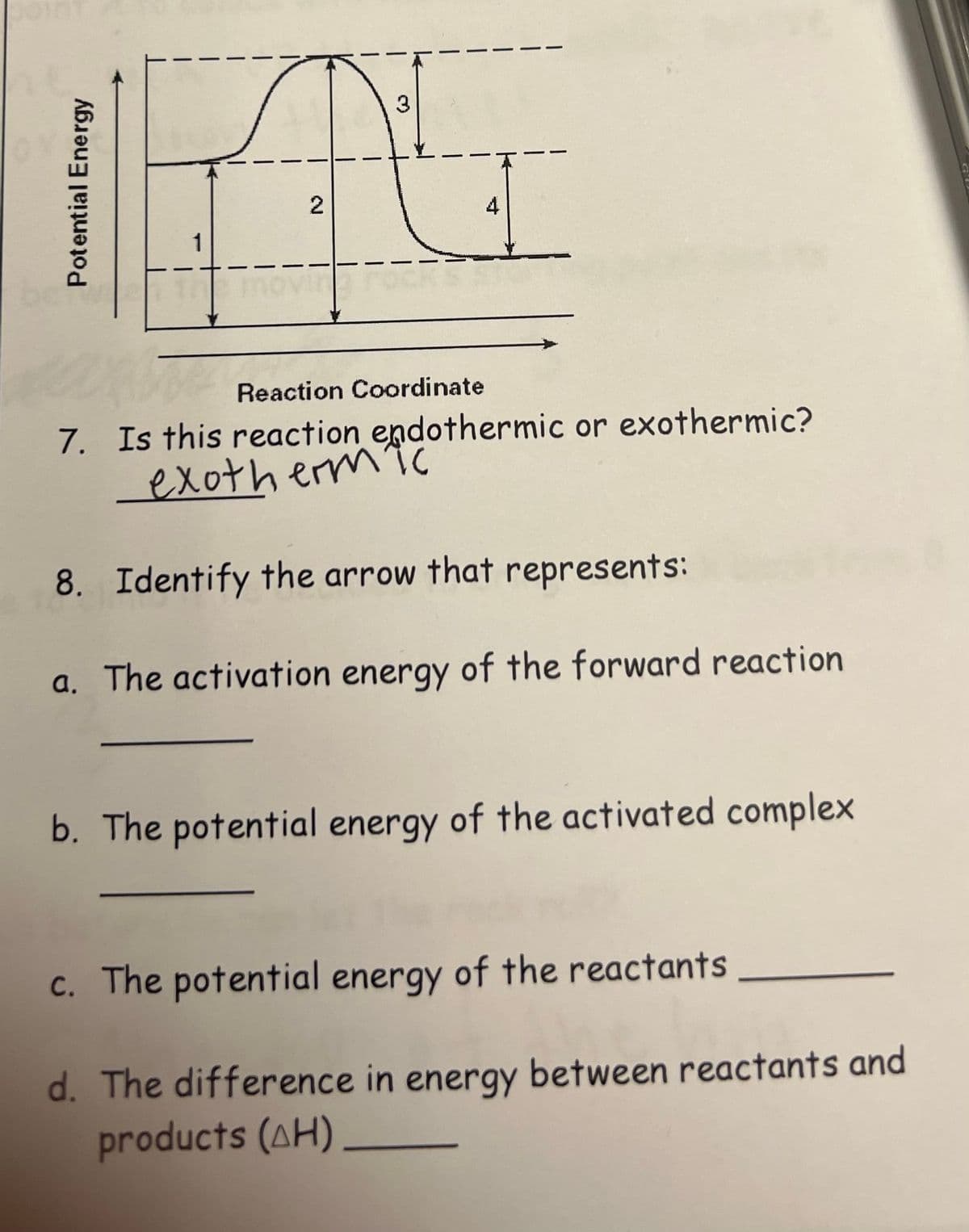
Transcribed Image Text:3
4
1
Reaction Coordinate
7. Is this reaction endothermic or exothermic?
exotherm
8. Identify the arrow that represents:
a. The activation energy of the forward reaction
b. The potential energy of the activated complex
c. The potential energy of the reactants
d. The difference in energy between reactants and
products (AH)
GU
2.
Potential Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax