3) Fluoridated teeth, CaF2 has a Ks.p. = 6.5 x 10-11. How many grams of Calcium would dissolve per 100 ml : (a) in pure water, (b) in a 0.75M AIF3 solution
3) Fluoridated teeth, CaF2 has a Ks.p. = 6.5 x 10-11. How many grams of Calcium would dissolve per 100 ml : (a) in pure water, (b) in a 0.75M AIF3 solution
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.42QAP
Related questions
Question
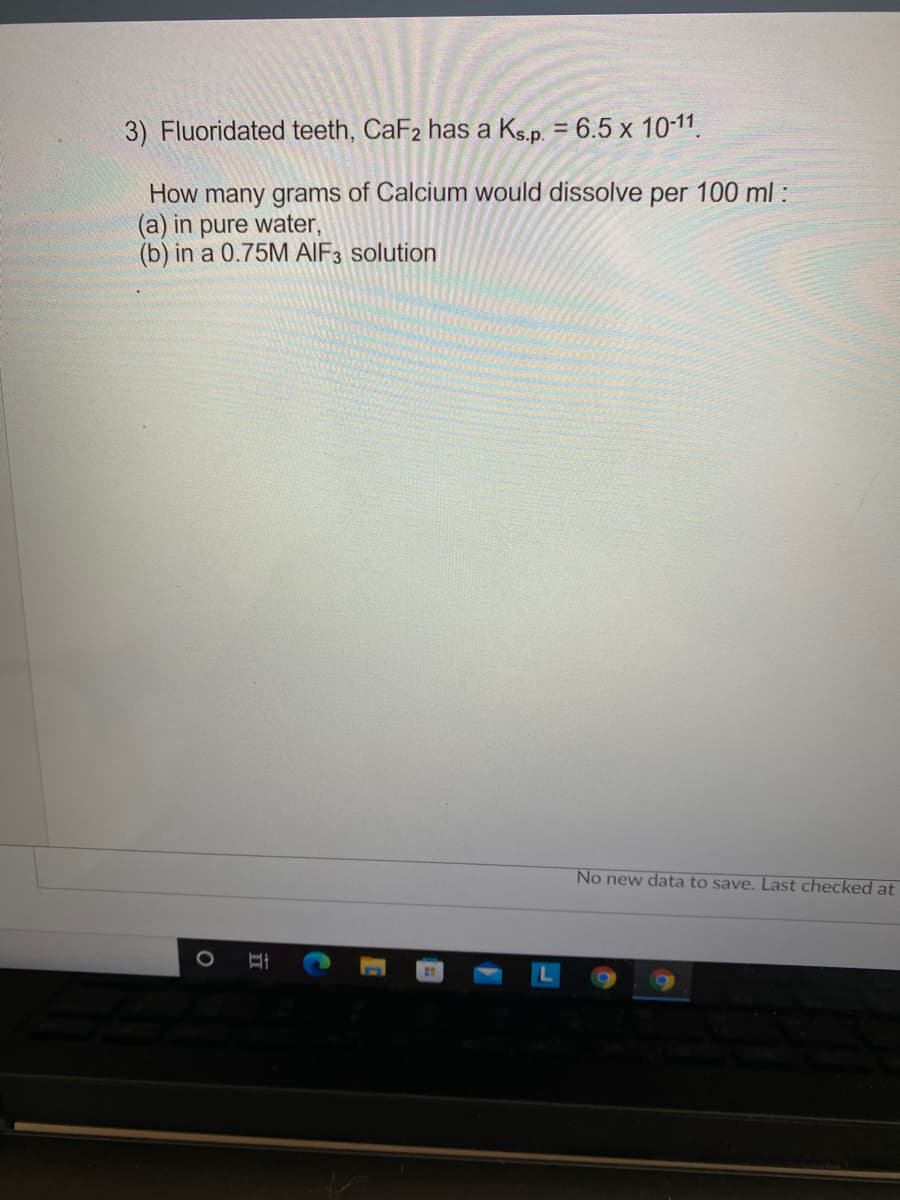
Transcribed Image Text:3) Fluoridated teeth, CaF2 has a Ks.p. = 6.5 x 10-11.
%3D
How many grams of Calcium would dissolve per 100 ml:
(a) in pure water,
(b) in a 0.75M AIF3 solution
No new data to save. Last checked at
Expert Solution
Step 1
is a sparingly soluble salt and can be partially dissociated as:
If the concentration of calcium fluoride is M, then we can have the following relation:
According to the definition of solubility product, we have
Again, we can write
This will be the final expression of solubility product for
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



