3) From the following data, calculate AH° (391.4 K) for the reaction CH3COOH) + 202 (a) → 2H20(9) + 2002 (9) Reaction A.H° (kJ/mol) CH3COOH) + 202 o) → 2H20(1) + 2C02 ca) 871.5 (at 298.15 к) H200→ H20(a) 40.656 (at 298.15 к) CH3COOH()→ CH3COOH) 24.4 (at 391.4 K) Substance CH;COOH (I) O2 (g) Co; (g) H;0 (1) H;0 (g) Cp.m/R 14.9 3.53 4.46 9.055 4.038
3) From the following data, calculate AH° (391.4 K) for the reaction CH3COOH) + 202 (a) → 2H20(9) + 2002 (9) Reaction A.H° (kJ/mol) CH3COOH) + 202 o) → 2H20(1) + 2C02 ca) 871.5 (at 298.15 к) H200→ H20(a) 40.656 (at 298.15 к) CH3COOH()→ CH3COOH) 24.4 (at 391.4 K) Substance CH;COOH (I) O2 (g) Co; (g) H;0 (1) H;0 (g) Cp.m/R 14.9 3.53 4.46 9.055 4.038
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter17: Chemcial Thermodynamics
Section: Chapter Questions
Problem 17.94QE: Suppose you have an endothermic reaction with H = + 15 kJ and a S of 150 J/K. Calculate G and Keq at...
Related questions
Question
! ( plz answer was with explanation)
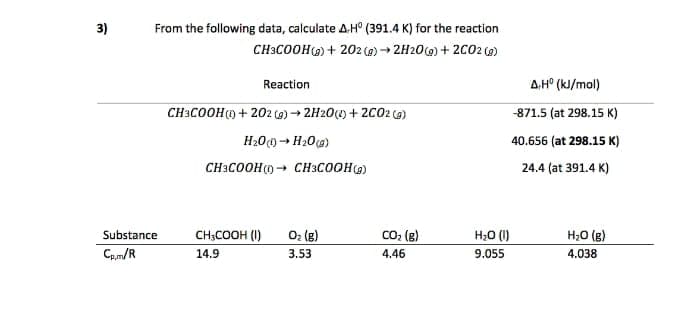
Transcribed Image Text:3)
From the following data, calculate AH° (391.4 K) for the reaction
CH3COOH) + 202 (g) → 2H20(9) + 2002 (9)
Reaction
A.H° (kJ/mol)
CH3COOH) + 202 6) → 2H20(1) + 2C02 ca)
871.5 (at 298.15 к)
H200→ H20 (a)
40.656 (at 298.15 к)
CH3COOH()→ CH3COOH()
24.4 (at 391.4 K)
Substance
CH;COOH (I)
O2 (g)
CO; (g)
H;0 (1)
H;0 (g)
Cp.m/R
14.9
3.53
4.46
9.055
4.038
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning