Oxidation of zinc Data: Standard molar enthalpy of formation of ZnCl2 (s): A¢H°1= - 416.12 kJ mol Standard molar enthalpy of formation of HCl (g): A¡H°2 = 92.08 kJ mol-! Standard molar enthalpy of dissolution of ZnCl2 (s): A;H°3= - 65.71 kJ mol- Standard molar enthalpy of dissolution of HCI (g): A¡H°4 = - 72.90 kJ mol-l Standard molar enthalpy of formation of H+ (aq): A;H°(H†, aq) = 0 kJ mol- Let's consider the reaction of oxidation of zinc by the solvated proton in the aqueous phase. This reaction is represented by the chemical reaction (1): (1) Zn (s) + 2H+ (aq) → Zn2+ (aq) + H2 (g) · Calculate the standard molar enthalpy for reaction (1). . Calculate the standard molar enthalpy of formation of the zinc cation Zn²+ in aqueous solution A,H°(Zn²*, aq).
Oxidation of zinc Data: Standard molar enthalpy of formation of ZnCl2 (s): A¢H°1= - 416.12 kJ mol Standard molar enthalpy of formation of HCl (g): A¡H°2 = 92.08 kJ mol-! Standard molar enthalpy of dissolution of ZnCl2 (s): A;H°3= - 65.71 kJ mol- Standard molar enthalpy of dissolution of HCI (g): A¡H°4 = - 72.90 kJ mol-l Standard molar enthalpy of formation of H+ (aq): A;H°(H†, aq) = 0 kJ mol- Let's consider the reaction of oxidation of zinc by the solvated proton in the aqueous phase. This reaction is represented by the chemical reaction (1): (1) Zn (s) + 2H+ (aq) → Zn2+ (aq) + H2 (g) · Calculate the standard molar enthalpy for reaction (1). . Calculate the standard molar enthalpy of formation of the zinc cation Zn²+ in aqueous solution A,H°(Zn²*, aq).
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 84AP
Related questions
Question
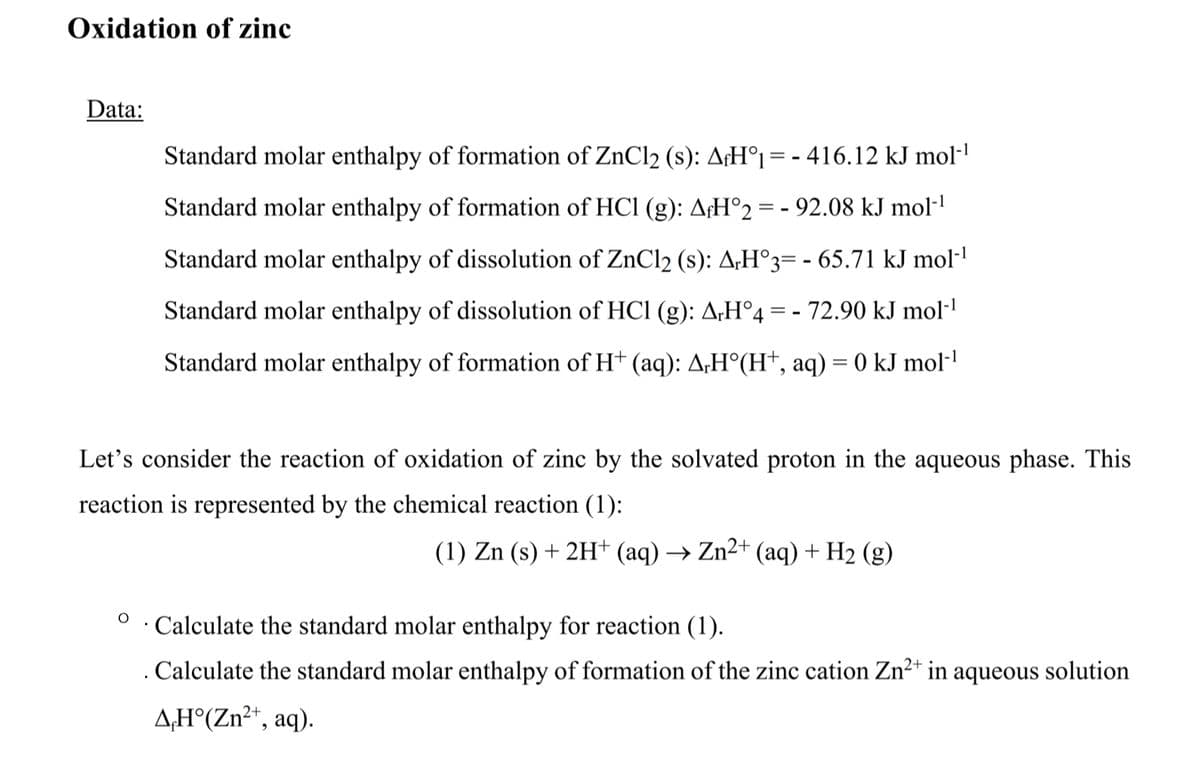
Transcribed Image Text:Oxidation of zinc
Data:
Standard molar enthalpy of formation of ZnCl2 (s): A¡H°1 = - 416.12 kJ mol-l
Standard molar enthalpy of formation of HCl (g): A¢H°2 = - 92.08 kJ mol·'
Standard molar enthalpy of dissolution of ZnCl2 (s): A;H°3= - 65.71 kJ mol-'
Standard molar enthalpy of dissolution of HCl (g): A;H°4 = - 72.90 kJ mol-'
Standard molar enthalpy of formation of H† (aq): A;H°(H†, aq) = 0 kJ mol·'
Let's consider the reaction of oxidation of zinc by the solvated proton in the aqueous phase. This
reaction is represented by the chemical reaction (1):
(1) Zn (s) + 2H+ (aq) → Zn²+ (aq) + H2 (g)
Calculate the standard molar enthalpy for reaction (1).
. Calculate the standard molar enthalpy of formation of the zinc cation Zn2* in aqueous solution
A;H°(Zn²*, aq).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning