3) In a tower 2 components, named as A and B, are present. The components are mutually present both in the gas and liquid phases. Component A, which is the solute, diffuses from the gas phase to the liquid phase. Component B, on the other hand, is the non-diffusing stagnant component. At a certain location in the tower, the bulk concentration of A in the gas phase is 45% by mole; and the bulk concentration of A in the liquid phase is 5% by mole. The film mass-transfer coefficients in the gas phase and the liquid phase are calculated as 1.465 x 10 kg mol A/s.m2.mol frac. and 1.967 x 10 kg mol A/s.m2.mol frac., respectively. Considering the given information, please calculate the interface concentration and the flux of A by using both liquid and gas phase equations. YA 0.00 0.02 0.05 0.09 0.13 0.18 0.25 0.37 0.53 0.72 XA 0.00 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40 0.45
3) In a tower 2 components, named as A and B, are present. The components are mutually present both in the gas and liquid phases. Component A, which is the solute, diffuses from the gas phase to the liquid phase. Component B, on the other hand, is the non-diffusing stagnant component. At a certain location in the tower, the bulk concentration of A in the gas phase is 45% by mole; and the bulk concentration of A in the liquid phase is 5% by mole. The film mass-transfer coefficients in the gas phase and the liquid phase are calculated as 1.465 x 10 kg mol A/s.m2.mol frac. and 1.967 x 10 kg mol A/s.m2.mol frac., respectively. Considering the given information, please calculate the interface concentration and the flux of A by using both liquid and gas phase equations. YA 0.00 0.02 0.05 0.09 0.13 0.18 0.25 0.37 0.53 0.72 XA 0.00 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40 0.45
Chapter93: Refractometry
Section: Chapter Questions
Problem 1P
Related questions
Question
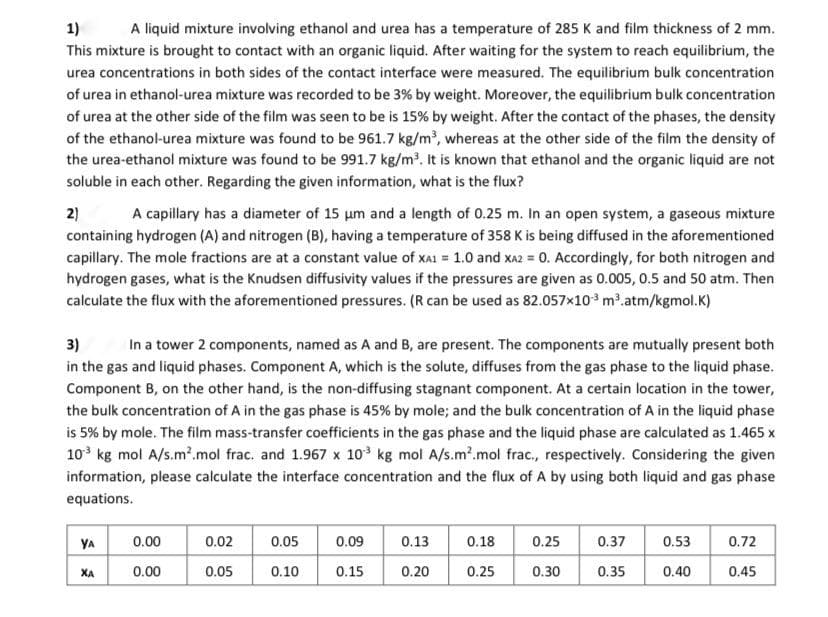
Transcribed Image Text:1)
A liquid mixture involving ethanol and urea has a temperature of 285 K and film thickness of 2 mm.
This mixture is brought to contact with an organic liquid. After waiting for the system to reach equilibrium, the
urea concentrations in both sides of the contact interface were measured. The equilibrium bulk concentration
of urea in ethanol-urea mixture was recorded to be 3% by weight. Moreover, the equilibrium bulk concentration
of urea at the other side of the film was seen to be is 15% by weight. After the contact of the phases, the density
of the ethanol-urea mixture was found to be 961.7 kg/m, whereas at the other side of the film the density of
the urea-ethanol mixture was found to be 991.7 kg/m?. It is known that ethanol and the organic liquid are not
soluble in each other. Regarding the given information, what is the flux?
2)
A capillary has a diameter of 15 um and a length of 0.25 m. In an open system, a gaseous mixture
containing hydrogen (A) and nitrogen (B), having a temperature of 358 K is being diffused in the aforementioned
capillary. The mole fractions are at a constant value of xAI = 1.0 and xa2 = 0. Accordingly, for both nitrogen and
hydrogen gases, what is the Knudsen diffusivity values if the pressures are given as 0.005, 0.5 and 50 atm. Then
calculate the flux with the aforementioned pressures. (R can be used as 82.057x10 m2.atm/kgmol.K)
3)
In a tower 2 components, named as A and B, are present. The components are mutually present both
in the gas and liquid phases. Component A, which is the solute, diffuses from the gas phase to the liquid phase.
Component B, on the other hand, is the non-diffusing stagnant component. At a certain location in the tower,
the bulk concentration of A in the gas phase is 45% by mole; and the bulk concentration of A in the liquid phase
is 5% by mole. The film mass-transfer coefficients in the gas phase and the liquid phase are calculated as 1.465 x
10 kg mol A/s.m2.mol frac. and 1.967 x 10 kg mol A/s.m².mol frac., respectively. Considering the given
information, please calculate the interface concentration and the flux of A by using both liquid and gas phase
equations.
YA
0.00
0.02
0.05
0.09
0.13
0.18
0.25
0.37
0.53
0.72
XA
0.00
0.05
0.10
0.15
0.20
0.25
0.30
0.35
0.40
0.45
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT