3 points Save Answer Consider the following reaction with vanadium and iron: 2 Fe²+ (aq) + √²+ (aq) 2 Fe3+ (aq) + V(s) When this reaction reaches equilibrium, what will be the cell potential (V)? Fe3+ + e → Fe2+ E = +0.77 V2+ + 2e → V E = -0.49 (the answer should be entered with 3 DIGITS; do not enter units; give answer in normal notation--examples include 0.00 and 1.23 and 120. and -123 and 123. and 12.3)
3 points Save Answer Consider the following reaction with vanadium and iron: 2 Fe²+ (aq) + √²+ (aq) 2 Fe3+ (aq) + V(s) When this reaction reaches equilibrium, what will be the cell potential (V)? Fe3+ + e → Fe2+ E = +0.77 V2+ + 2e → V E = -0.49 (the answer should be entered with 3 DIGITS; do not enter units; give answer in normal notation--examples include 0.00 and 1.23 and 120. and -123 and 123. and 12.3)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter17: Electrochemistry And Its Applications
Section: Chapter Questions
Problem 35QRT
Related questions
Question
10
Transcribed Image Text:Save Answer
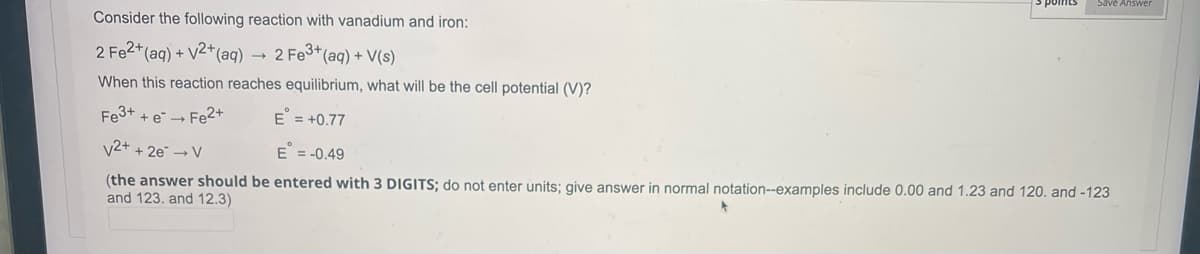
Consider the following reaction with vanadium and iron:
2 Fe2+ (aq) + √2+ (aq)
2 Fe3+ (aq) + V(s)
When this reaction reaches equilibrium, what will be the cell potential (V)?
Fe3+ + e → Fe2+
E = +0.77
V2+ + 2e → V
E = -0.49
(the answer should be entered with 3 DIGITS; do not enter units; give answer in normal notation--examples include 0.00 and 1.23 and 120. and -123
and 123. and 12.3)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning