3- The bonds that would be expected for atoms forming H2O molecule and joining H2O molecules respectively are: a) hydrogen and covalent b) covalent and hydrogen c) induced dipole and hydrogen d) covalent and induced dipole
3- The bonds that would be expected for atoms forming H2O molecule and joining H2O molecules respectively are: a) hydrogen and covalent b) covalent and hydrogen c) induced dipole and hydrogen d) covalent and induced dipole
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter3: Electron Orbitals
Section: Chapter Questions
Problem 12CTQ
Related questions
Question
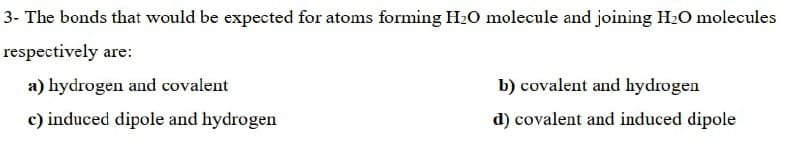
Transcribed Image Text:3- The bonds that would be expected for atoms forming H2O molecule and joining H2O molecules
respectively are:
a) hydrogen and covalent
b) covalent and hydrogen
c) induced dipole and hydrogen
d) covalent and induced dipole
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry In Focus
Chemistry
ISBN:
9781305084476
Author:
Tro, Nivaldo J., Neu, Don.
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry In Focus
Chemistry
ISBN:
9781305084476
Author:
Tro, Nivaldo J., Neu, Don.
Publisher:
Cengage Learning