3. 1986 B Three volatile compounds X, Y, and Z each contain element Q. The percent by weight of element Q in each compound was determined. Some of the data obtained are given below. Percent by Weight of Element Q Compound Molecular Weight 64.8% Y 73.0% 104. Z 59.3% 64.0 The vapor density of compound X at 27°C and 750. mm Hg was determined to be 3.53 grams per litre. Calculate the molecular weight of compound X. Determine the mass of element Q contained in 1.00 mole of each of the three compounds. Calculate the most probable value of the atomic weight of element Q. Compound Z contains carbon, hydrogen, and element Q. When 1.00 gram of compound Z is oxidized and all of the carbon and hydrogen are converted to oxides, 1.37 grams of CO, and 0.281 gram of water are produced. Determine the most probable molecular formula of compound Z. а. b. с. d.
3. 1986 B Three volatile compounds X, Y, and Z each contain element Q. The percent by weight of element Q in each compound was determined. Some of the data obtained are given below. Percent by Weight of Element Q Compound Molecular Weight 64.8% Y 73.0% 104. Z 59.3% 64.0 The vapor density of compound X at 27°C and 750. mm Hg was determined to be 3.53 grams per litre. Calculate the molecular weight of compound X. Determine the mass of element Q contained in 1.00 mole of each of the three compounds. Calculate the most probable value of the atomic weight of element Q. Compound Z contains carbon, hydrogen, and element Q. When 1.00 gram of compound Z is oxidized and all of the carbon and hydrogen are converted to oxides, 1.37 grams of CO, and 0.281 gram of water are produced. Determine the most probable molecular formula of compound Z. а. b. с. d.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter20: Chemistry Of The Metals
Section: Chapter Questions
Problem 36QAP: A 0.500-g sample of zinc-copper alloy was treated with dilute hydrochloric acid. The hydrogen gas...
Related questions
Question
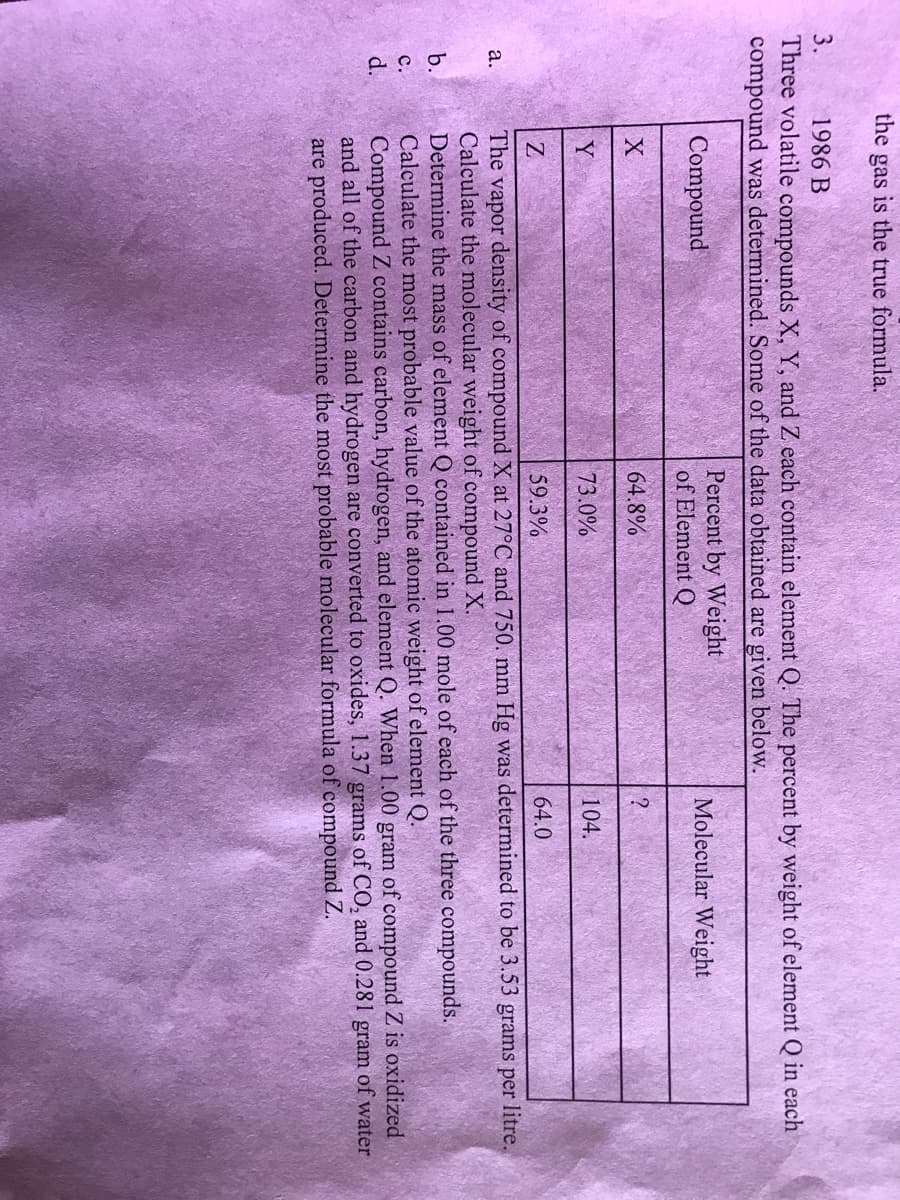
Transcribed Image Text:3.
1986 B
Three volatile compounds X, Y, and Z each contain element Q. The percent by weight of element Q in each
compound was determined. Some of the data obtained are given below.
Percent by Weight
of Element Q
Compound
Molecular Weight
64.8%
Y
73.0%
104.
Z
59.3%
64.0
The vapor density of compound X at 27°C and 750. mm Hg was determined to be 3.53 grams per litre.
Calculate the molecular weight of compound X.
Determine the mass of element Q contained in 1.00 mole of each of the three compounds.
Calculate the most probable value of the atomic weight of element Q.
Compound Z contains carbon, hydrogen, and element Q. When 1.00 gram of compound Z is oxidized
and all of the carbon and hydrogen are converted to oxides, 1.37 grams of CO, and 0.281 gram of water
are produced. Determine the most probable molecular formula of compound Z.
а.
b.
с.
d.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning