() The student finds the following particulate model of AgCI(s). Assuming the crystal structures are similar, how should the student modify the model to represent AgBr(s)? Justify your answer in terms of the radius and arrangement of electrons in the ions.
() The student finds the following particulate model of AgCI(s). Assuming the crystal structures are similar, how should the student modify the model to represent AgBr(s)? Justify your answer in terms of the radius and arrangement of electrons in the ions.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter9: Chemical Bonds
Section: Chapter Questions
Problem 9.102QE
Related questions
Question
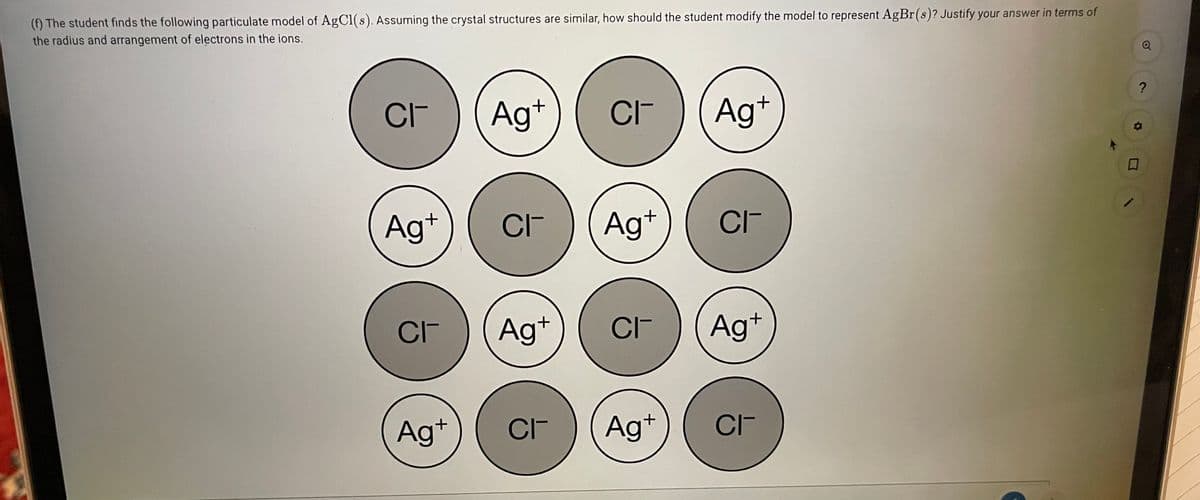
Transcribed Image Text:(f) The student finds the following particulate model of AgCl(s). Assuming the crystal structures are similar, how should the student modify the model to represent AgBr(s)? Justify your answer in terms of
the radius and arrangement of electrons in the ions.
CI-
Ag+
CIF
Ag*
口
Ag+
CI-
Ag*
CIT
CI-
Ag+
CI-
Ag+
Ag+
CI-
Ag+
CIT
Transcribed Image Text:(g) The enthalpy of dissolution of AgCl(s) in water is a positive value. Based on this information, the student claims that the energy released when separating the ions in AgCl(s)is greater than the energy
released during the hydration of Ag+ and Cl by water molecules. Do you agree or disagree with the student's claim? Justify your answer.
B IU
x? X2 5 ¢ 2
三
0/10000 Word Limit
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
