Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter16: Acids And Bases
Section: Chapter Questions
Problem 65AP: . The concepts of acid-base equilibria were developed in this chapter for aqueous solutions (in...
Related questions
Question
Need help with number 3
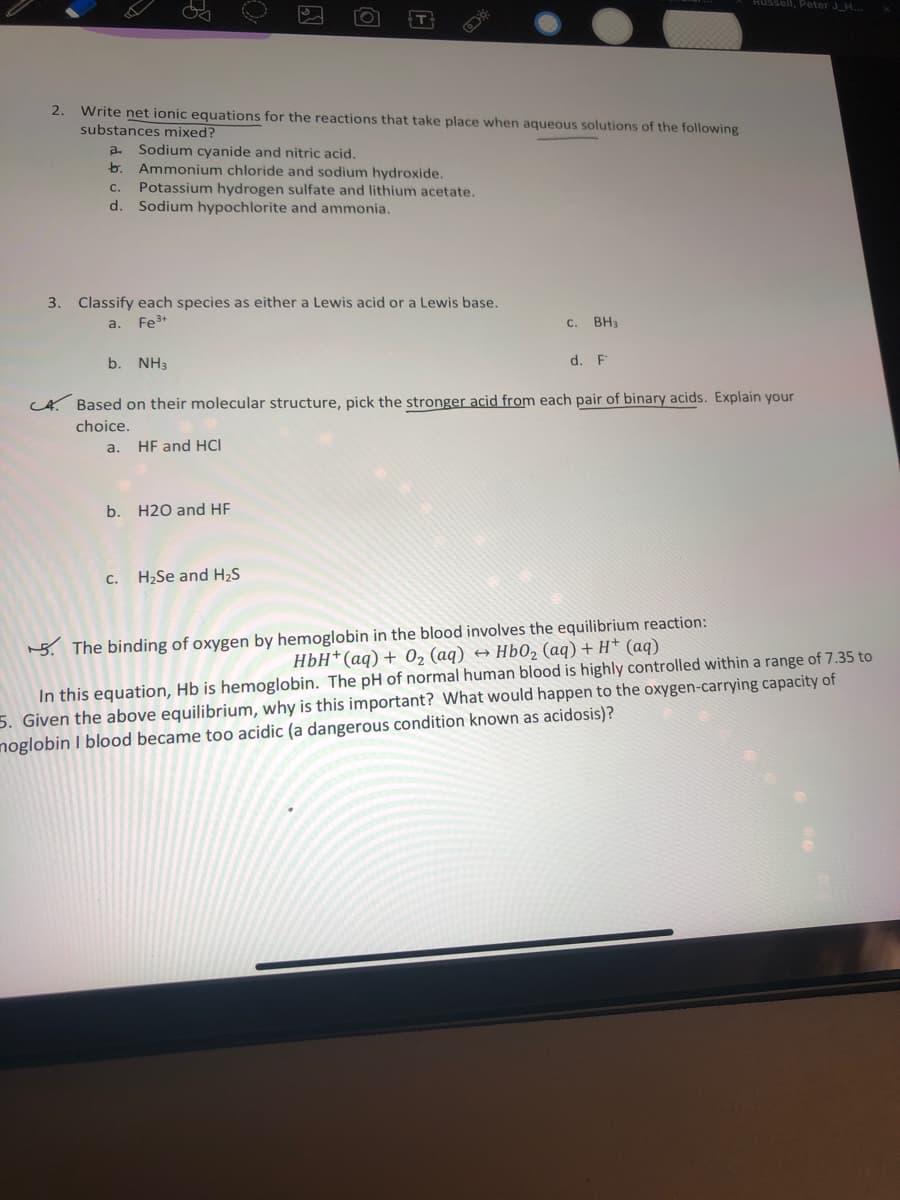
Transcribed Image Text:Russell, Peter J_H...
2.
Write net ionic equations for the reactions that take place when aqueous solutions of the following
substances mixed?
Sodium cyanide and nitric acid.
b. Ammonium chloride and sodium hydroxide.
a.
C.
Potassium hydrogen sulfate and lithium acetate.
d.
Sodium hypochlorite and ammonia.
3. Classify each species as either a Lewis acid or a Lewis base.
a. Fe3+
C. BH3
b. NH3
d. F
A. Based on their molecular structure, pick the stronger acid from each pair of binary acids. Explain your
choice.
a.
HF and HCI
b. H20 and HF
c. H2Se and H2S
5. The binding of oxygen by hemoglobin in the blood involves the equilibrium reaction:
HbH*(aq) + 02 (aq) → Hb0, (aq) + H* (aq)
In this equation, Hb is hemoglobin. The pH of normal human blood is highly controlled within a range of 7.35 to
5. Given the above equilibrium, why is this important? What would happen to the oxygen-carrying capacity of
noglobin I blood became too acidic (a dangerous condition known as acidosis)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax