Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter3: Chemical Bonds
Section: Chapter Questions
Problem 3.116P
Related questions
Question
Do question 3 and if you can do 4 too please and please make your handwriting clear
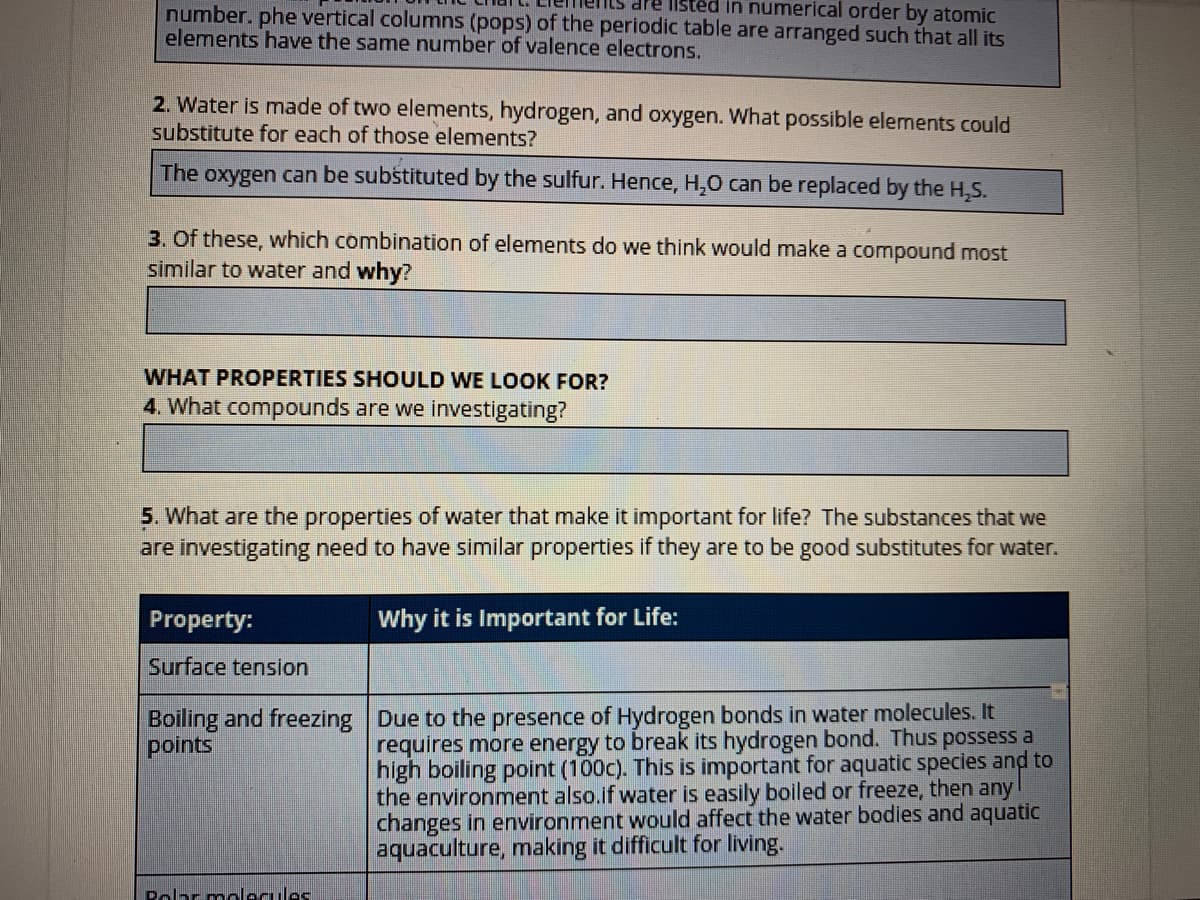
Transcribed Image Text:dre listed in numerical order by atomic
number. phe vertical columns (pops) of the periodic table are arranged such that all its
elements have the same number of valence electrons.
2. Water is made of two elements, hydrogen, and oxygen. What possible elements could
substitute for each of those elements?
The oxygen can be substituted by the sulfur. Hence, H,0 can be replaced by the H,S.
3. Of these, which combination of elements do we think would make a compound most
similar to water and why?
WHAT PROPERTIES SHOULD WE LOOK FOR?
4. What compounds are we investigating?
5. What are the properties of water that make it important for life? The substances that we
are investigating need to have similar properties if they are to be good substitutes for water.
Property:
Why it is Important for Life:
Surface tension
Boiling and freezing Due to the presence of Hydrogen bonds in water molecules. It
points
requires more energy to break its hydrogen bond. Thus possess a
high boiling point (100c). This is important for aquatic species and to
the environment also.if water is easily boiled or freeze, then any
changes in environment would affect the water bodies and aquatic
aquaculture, making it difficult for living.
Polar molecules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

