3. There is a class of polyesters that have the general structure shown below. . For example, in polyethylene terephthalate (PET) m =1. How would you expect the Tg ad Tm of these polymers to vary with m, given that the molecular weight of the chain is held constant? Give reasons for your answer. Also, assuming that you are now just considering one member of this class of polymers, say PET, how does the Tg vary with molecular weight, n.
3. There is a class of polyesters that have the general structure shown below. . For example, in polyethylene terephthalate (PET) m =1. How would you expect the Tg ad Tm of these polymers to vary with m, given that the molecular weight of the chain is held constant? Give reasons for your answer. Also, assuming that you are now just considering one member of this class of polymers, say PET, how does the Tg vary with molecular weight, n.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter8: Haloalkanes, Halogenation, And Radical Reactions
Section: Chapter Questions
Problem 8.34P
Related questions
Question
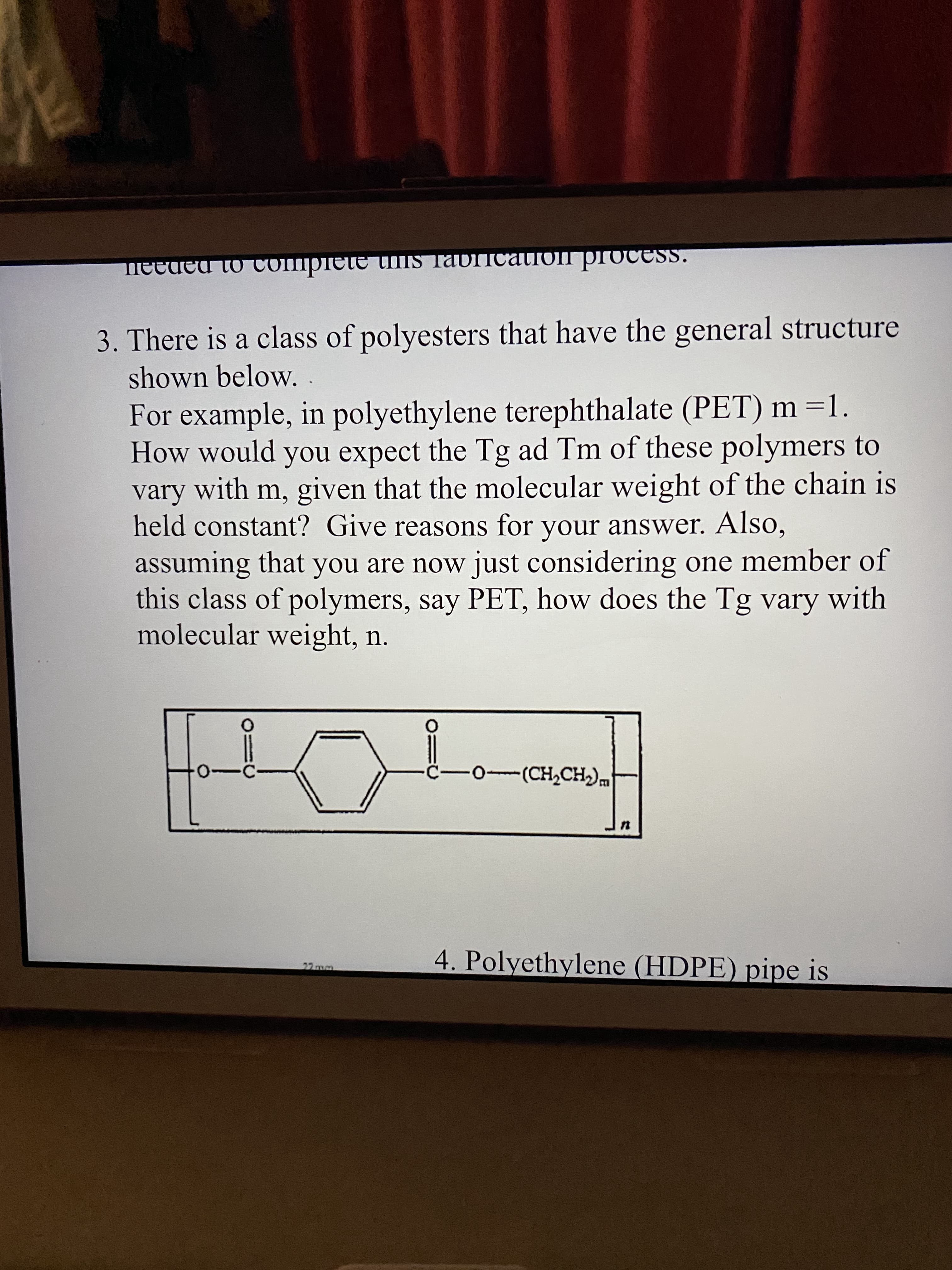
Transcribed Image Text:3. There is a class of polyesters that have the general structure
shown below. .
For example, in polyethylene terephthalate (PET) m =1.
How would you expect the Tg ad Tm of these polymers to
vary with m, given that the molecular weight of the chain is
held constant? Give reasons for your answer. Also,
assuming that you are now just considering one member of
this class of polymers, say PET, how does the Tg vary with
molecular weight, n.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning