3. Tina bought a drink that consists of 2.00 g aspartame (C14H18N2O5) dissolved in 250 g H20. Aspartame is an artificial, non-saccharide sweeter used as a sugar substitute in some foods and beverages. It is approximately 200 times sweeter than sucrose (table sugar). What is the molality of the aspartame solution?
3. Tina bought a drink that consists of 2.00 g aspartame (C14H18N2O5) dissolved in 250 g H20. Aspartame is an artificial, non-saccharide sweeter used as a sugar substitute in some foods and beverages. It is approximately 200 times sweeter than sucrose (table sugar). What is the molality of the aspartame solution?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter10: Properties Of Solutions
Section: Chapter Questions
Problem 129CP: In some regions of the southwest United States, the water is very hard. For example, in Las Cruces,...
Related questions
Question
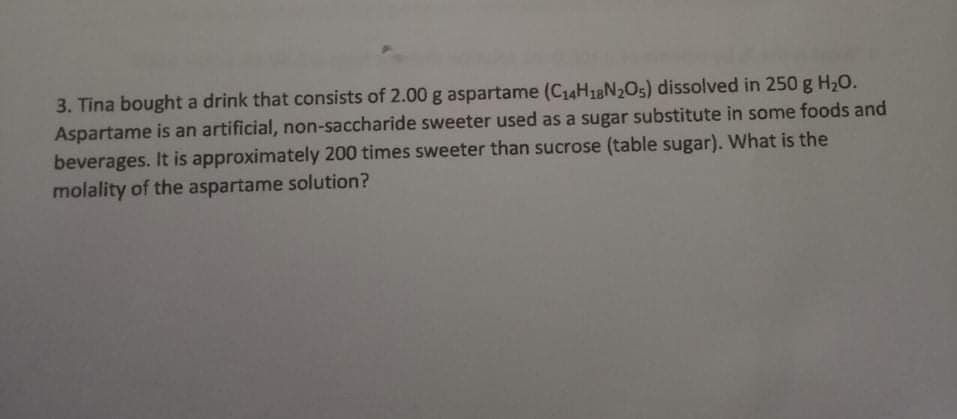
Transcribed Image Text:3. Tina bought a drink that consists of 2.00 g aspartame (C14H18N2O5) dissolved in 250 g H20.
Aspartame is an artificial, non-saccharide sweeter used as a sugar substitute in some foods and
beverages. It is approximately 200 times sweeter than sucrose (table sugar). What is the
molality of the aspartame solution?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning