3. Use your experimentalAT values to answer the following questions. (a) In which compound does your data suggest that the intermolecular attractive forces are the strongest? Does your data agree with the expected result? Explain (b) In which compound does your data suggest that the intermolecular attractive forces are the weakest? Does your data agree with the expected result? Explain
3. Use your experimentalAT values to answer the following questions. (a) In which compound does your data suggest that the intermolecular attractive forces are the strongest? Does your data agree with the expected result? Explain (b) In which compound does your data suggest that the intermolecular attractive forces are the weakest? Does your data agree with the expected result? Explain
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter12: States Of Matter
Section: Chapter Questions
Problem 5STP
Related questions
Question
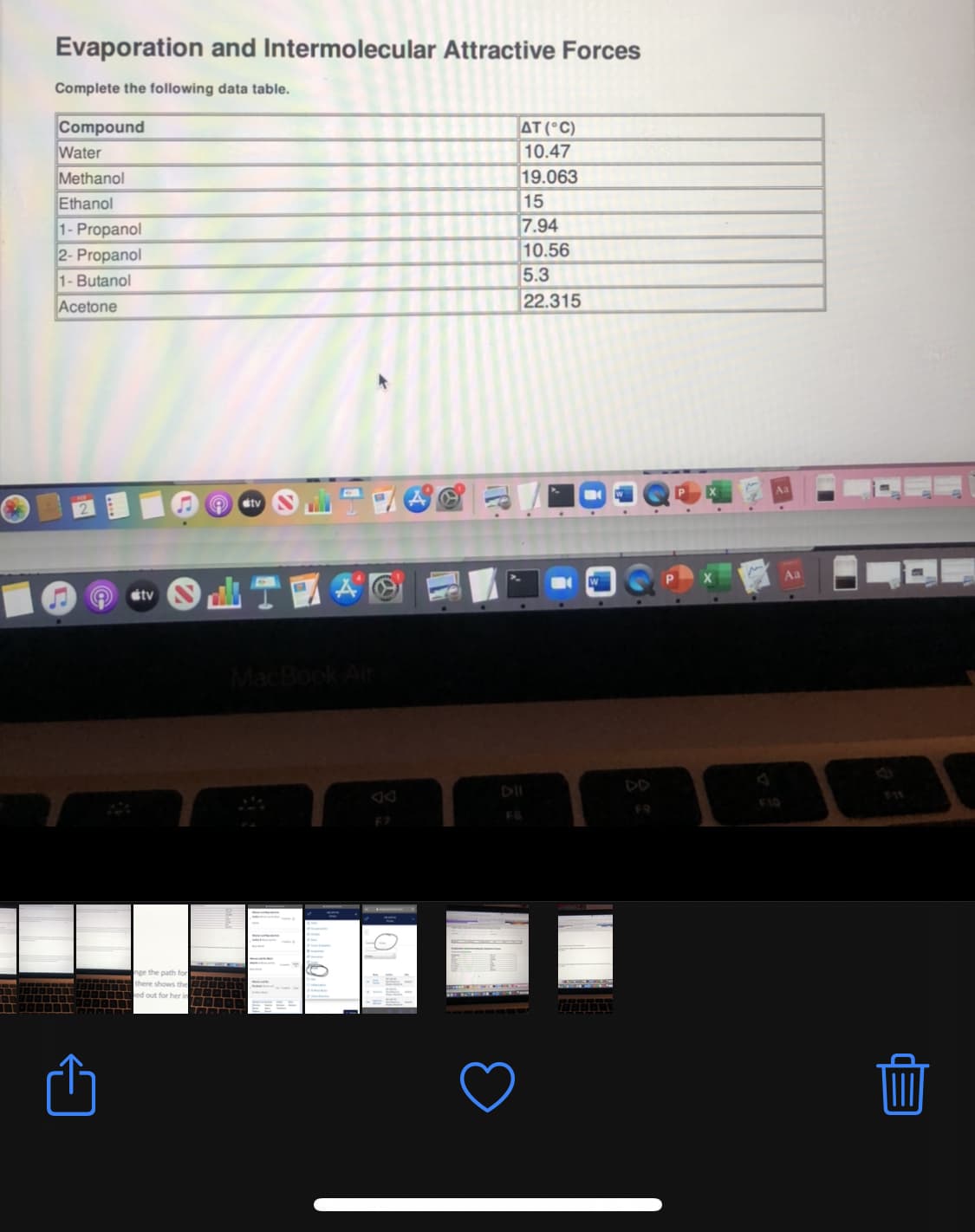
Transcribed Image Text:Evaporation and Intermolecular Attractive Forces
Complete the following data table.
Compound
Water
AT (°C)
10.47
19.063
15
7.94
10.56
5.3
Methanol
Ethanol
1- Propanol
2- Propanol
1- Butanol
Acetone
22.315
tv
Aa
stv
MacBook Air
DD
F10
F9
FS
F7
nge the path for
there shows the
ed out for her in
Transcribed Image Text:Wed 8:38 PM
!!
of
Screen Shot 2022-02-02 at 2.20.05 PM
Screen Shot 2022-02-02 at 2.23.36 PM -
Q Search
There is not enough space to save this document to
iCloud. Purchase additional storage or remove some
73%
Wed 2:23 PM
Tools
Window
Help
PM
documents from iCloud.
Week 3 Report Evap and IMF.pdf (page 2 of 3)
Edited
Q Search
PM
3. Use your experimental AT values to answer the following questions.
(a) In which compound does your data suggest that the intermolecular attractive forces are the strongest? Does your data agree
with the expected result? Explain
PM
PM
PM
(b) In which compound does your data suggest that the intermolecular attractive forces are the weakest? Does your data agree
with the expected result? Explain
PM
PM
931
stv
Aa
2.
工P(
stv
Aa
X
立工PO
MacBook Air
23
OLA
&
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning