3. When aqueous solutions of NH&OH and Cu(NO3)2 are mixed, a bluish precipitate forrms. What is the identity of this precipitate? b. Cu(ОН)2 d. CuOH c. NH4(NOs)2 a. NH4NOs
3. When aqueous solutions of NH&OH and Cu(NO3)2 are mixed, a bluish precipitate forrms. What is the identity of this precipitate? b. Cu(ОН)2 d. CuOH c. NH4(NOs)2 a. NH4NOs
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 78QAP: When 85.0 mL of 0.250 M Ba(OH)2 solution is added to 85.00 mL of 0.250 M Al (NO3)3 solution, a white...
Related questions
Question
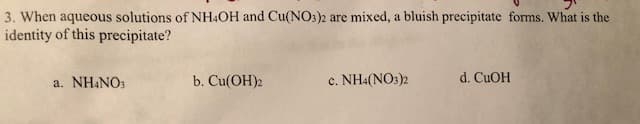
Transcribed Image Text:3. When aqueous solutions of NH&OH and Cu(NO3)2 are mixed, a bluish precipitate forrms. What is the
identity of this precipitate?
b. Cu(ОН)2
d. CuOH
c. NH4(NOs)2
a. NH4NOs
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning