3. Which polymer has higher Tg? 0 0 0 || || || || -C(CH₂)4CO(CH2)40- -C(CH₂)4CNH(CH₂)6NH- (A VS. B) (C VS. D) 5. Which of following monomers is most difficult to get high molecular weight polymer via radical polymerization using benzoyl peroxide as an initiator at 80 °C ? A. Acrylonitrile B. a-Methyl styrene C. Vinyl acetate D. 2-Hydroxyethyl methacrylate E. Tetrafluoroethylene +CH₂-CH+ n O O=C CH3 tam-GH+ C₂ OCH 3 =0
3. Which polymer has higher Tg? 0 0 0 || || || || -C(CH₂)4CO(CH2)40- -C(CH₂)4CNH(CH₂)6NH- (A VS. B) (C VS. D) 5. Which of following monomers is most difficult to get high molecular weight polymer via radical polymerization using benzoyl peroxide as an initiator at 80 °C ? A. Acrylonitrile B. a-Methyl styrene C. Vinyl acetate D. 2-Hydroxyethyl methacrylate E. Tetrafluoroethylene +CH₂-CH+ n O O=C CH3 tam-GH+ C₂ OCH 3 =0
Chapter31: Synthetic Polymers
Section31.SE: Something Extra
Problem 33AP
Related questions
Question
choose your answer and explain why?
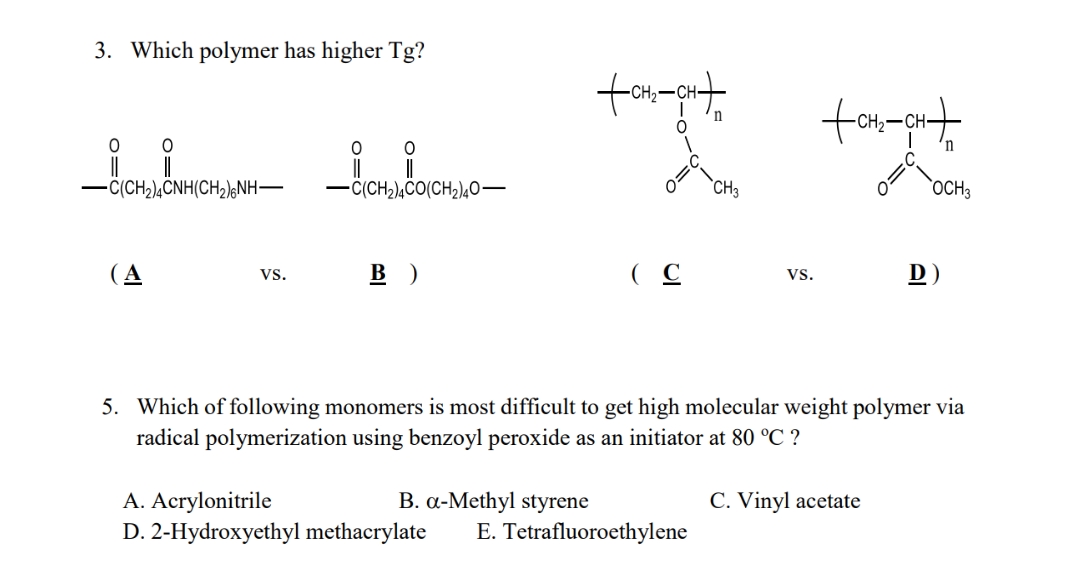
Transcribed Image Text:3. Which polymer has higher Tg?
0
0 0
-C(CH₂)4CNH(CH₂)6NH-
-C(CH₂)4CO(CH₂)40-
(A
VS.
B)
(C
VS.
D)
5. Which of following monomers is most difficult to get high molecular weight polymer via
radical polymerization using benzoyl peroxide as an initiator at 80 °C ?
A. Acrylonitrile
B. a-Methyl styrene
C. Vinyl acetate
D. 2-Hydroxyethyl methacrylate E. Tetrafluoroethylene
+CH₂-CH+
n
CH3
+CH₂-CH+
OCH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
