3.48 Determine the empirical formulas of the compounds with the following compositions by mass: (a) 42.1% Na, 18.9% P, and 39.0% O (b) 18.7% Li, 16.3% C, and 65.0% O (c) 60.0% C, 4.4% H, and the remainder O
3.48 Determine the empirical formulas of the compounds with the following compositions by mass: (a) 42.1% Na, 18.9% P, and 39.0% O (b) 18.7% Li, 16.3% C, and 65.0% O (c) 60.0% C, 4.4% H, and the remainder O
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter3: Equation, The Mole, And Chemical Formulas
Section: Chapter Questions
Problem 3.99QE
Related questions
Question
only a) plz!
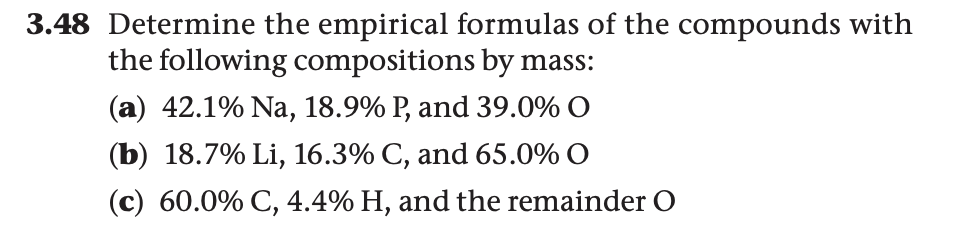
Transcribed Image Text:3.48 Determine the empirical formulas of the compounds with
the following compositions by mass:
(a) 42.1% Na, 18.9% P, and 39.0% O
(b) 18.7% Li, 16.3% C, and 65.0% O
(c) 60.0% C, 4.4% H, and the remainder O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning