31) The following energy diagram shows the relative energies of the conformations of butane produced during the rotation about the C2-C3 bond. Which of the following structure represents the lowest energy conformer? Energy Mw T T T 60 T 180 T 300 T 360 120 240 H CH3 H H H H. H H H3C- pa H H3C H H₂d²C CH3 H A B C D E 32) Which of the following is the most stable conformer of trans-1-isopropyl-3-methylcyclohexane? JAAN A B C AY E) None of above D H₂C₂ H H H CH3 H₂C H H TH
31) The following energy diagram shows the relative energies of the conformations of butane produced during the rotation about the C2-C3 bond. Which of the following structure represents the lowest energy conformer? Energy Mw T T T 60 T 180 T 300 T 360 120 240 H CH3 H H H H. H H H3C- pa H H3C H H₂d²C CH3 H A B C D E 32) Which of the following is the most stable conformer of trans-1-isopropyl-3-methylcyclohexane? JAAN A B C AY E) None of above D H₂C₂ H H H CH3 H₂C H H TH
Chapter3: Organic Compounds: Alkanes And Their Stereochemistry
Section3.SE: Something Extra
Problem 46AP: Draw the most stable conformation of pentane, using wedges and dashes to represent bonds coming out...
Related questions
Question
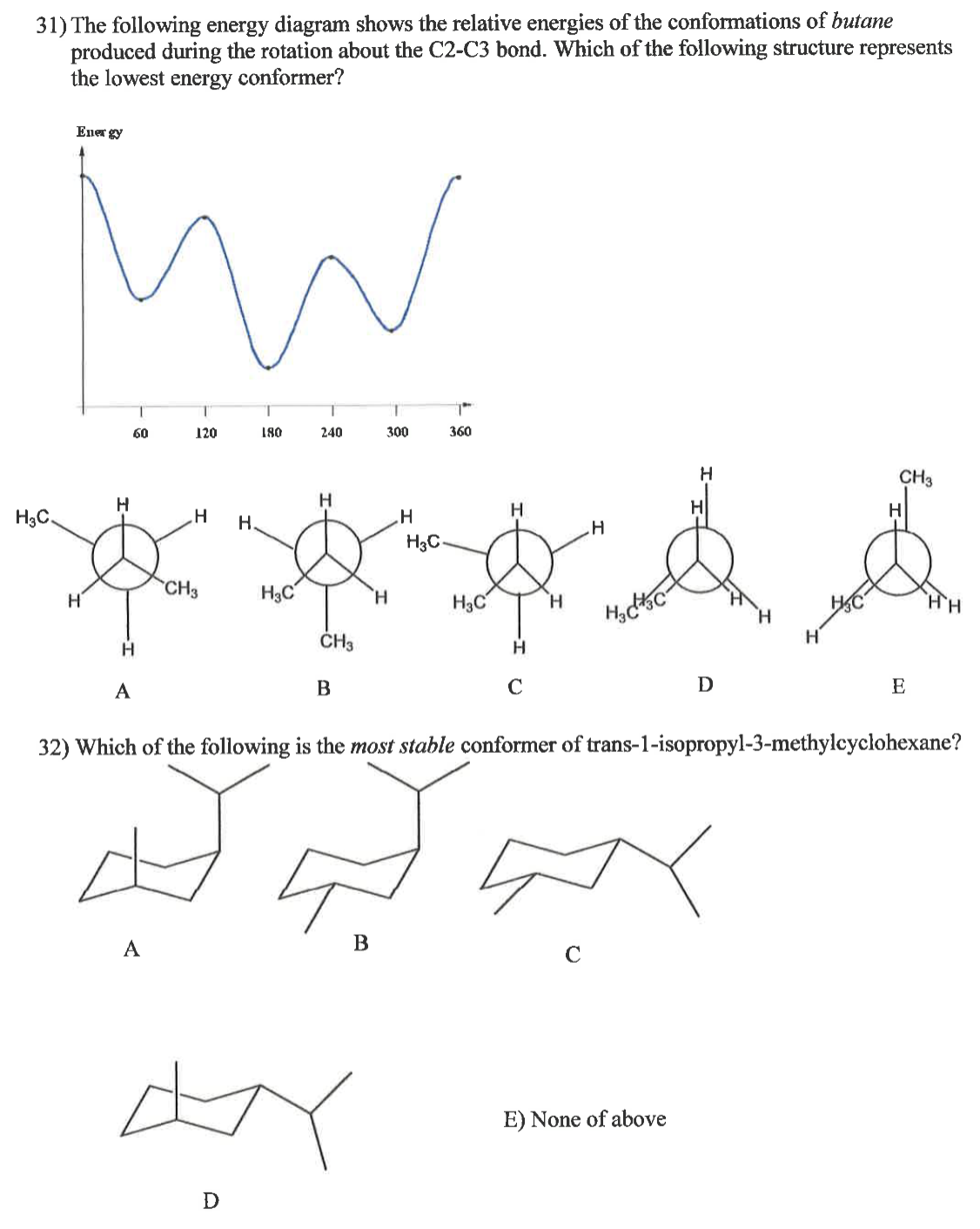
Transcribed Image Text:31) The following energy diagram shows the relative energies of the conformations of butane
produced during the rotation about the C2-C3 bond. Which of the following structure represents
the lowest energy conformer?
Energy
in
T
T
T
T
120
60
180
240
300
360
H
CH3
H
H
H
H
H
H₂C.
H
H.
H
H
H3C
xofa d
CH3
H₂C
H
H
H3C
H
HH
CH3
H
D
C
B
A
E
32) Which of the following is the most stable conformer of trans-1-isopropyl-3-methylcyclohexane?
B
A
E) None of above
D
יד
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning