Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter9: Gases
Section: Chapter Questions
Problem 80E: One method of analyzing amino acids is the van Slyke method. The characteristic amino groups (NH2)...
Related questions
Question
Question 33 is the question I’m stuck one
Transcribed Image Text:test.html?rowid%3D1188938#question33
Reading
ruler
Magni
fier
Highlight
mode
Q Zoom
Q Zo
out
<O >
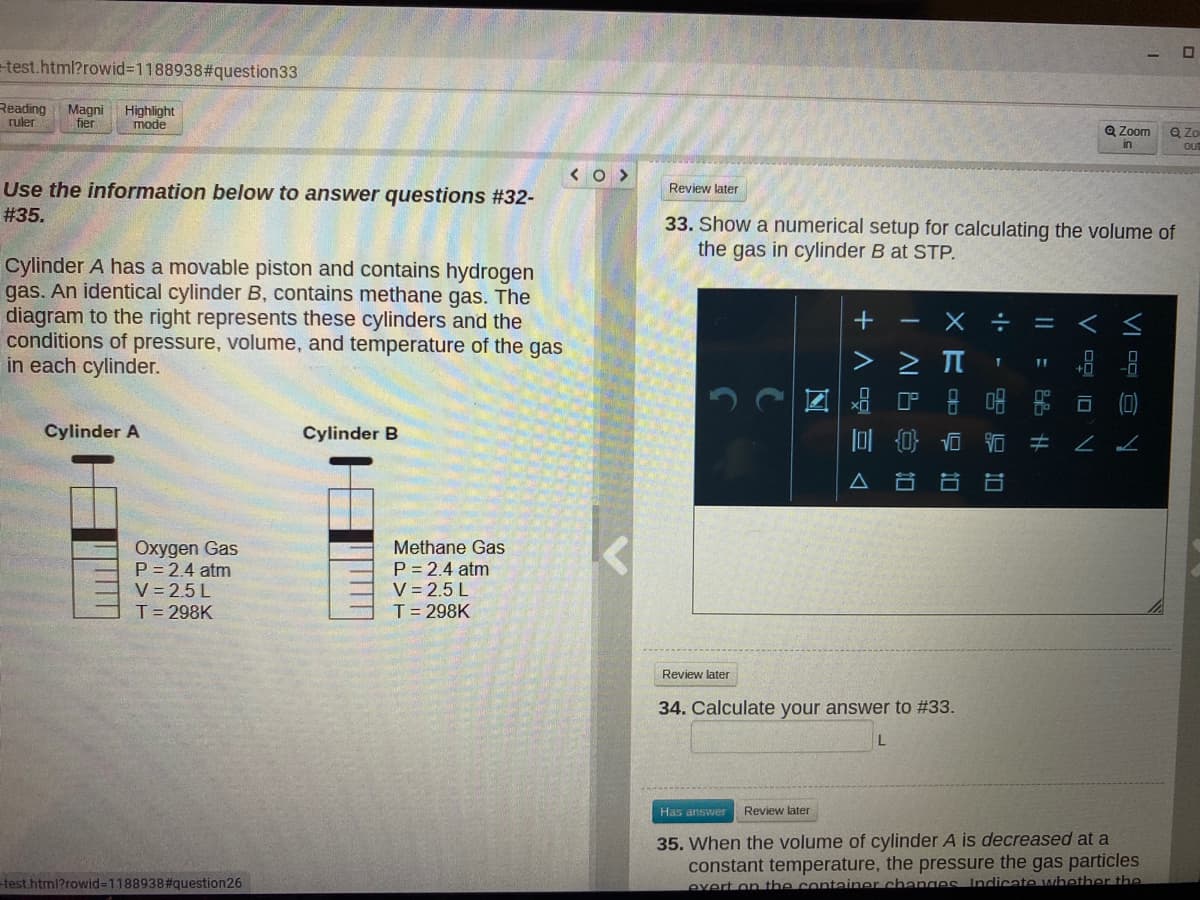
Use the information below to answer questions #32-
# 35.
Review later
33. Show a numerical setup for calculating the volume of
the gas in cylinder B at STP.
Cylinder A has a movable piston and contains hydrogen
gas. An identical cylinder B, contains methane gas. The
diagram to the right represents these cylinders and the
conditions of pressure, volume, and temperature of the gas
in each cylinder.
回 P 8 唱可
Cylinder A
Cylinder B
回卓 白
A 首台 台
Oxygen Gas
P = 2.4 atm
V = 2.5 L
T= 298K
Methane Gas
P = 2.4 atm
V = 2.5 L
T= 298K
Review later
34. Calculate your answer to #33.
Has answer
Review later
35. When the volume of cylinder A is decreased at a
constant temperature, the pressure the gas particles
exert on the container changes Indicate whether the
test.html?rowid31188938#question26
|| =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning