35. Which of the following concepts can be used to explain the difference in acidity between acetylene (C2H2) and ethylene (C2H4)? A) Size B) Resonance C) Inductive effect D) Hybridization 36. Which of the following concepts can be used to explain the difference in acidity between ethanol (CH;CH2OH) and 2-fluoroethanol (FCH2CH2OH)? A) Size B) Inductive effect C) Resonance D) Hybridization
35. Which of the following concepts can be used to explain the difference in acidity between acetylene (C2H2) and ethylene (C2H4)? A) Size B) Resonance C) Inductive effect D) Hybridization 36. Which of the following concepts can be used to explain the difference in acidity between ethanol (CH;CH2OH) and 2-fluoroethanol (FCH2CH2OH)? A) Size B) Inductive effect C) Resonance D) Hybridization
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter16: Amines
Section: Chapter Questions
Problem 16.17P: 16-17 Propylamine (bp 48°C), ethylmethylamine (bp 37°C), and trimethylamine (bp 3°C) are...
Related questions
Question
please answer the 2 questions please
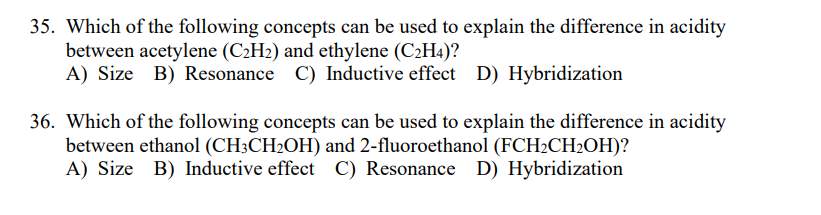
Transcribed Image Text:35. Which of the following concepts can be used to explain the difference in acidity
between acetylene (C2H2) and ethylene (C2H4)?
A) Size B) Resonance C) Inductive effect D) Hybridization
36. Which of the following concepts can be used to explain the difference in acidity
between ethanol (CH3CH2OH) and 2-fluoroethanol (FCH2CH2OH)?
A) Size B) Inductive effect C) Resonance D) Hybridization
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning
