Q: 26) Predict the geometry about the first carbon atom (on the left), determine the hybridization type…
A: Given : Structure of Propene To find : Hybridization and Geometry of double bonded carbon in which…
Q: 1. Answer the following questions regarding compound A. а. How many carbons and how many hydrogens…
A: Solution: The chemical structure of the given compound is shown below.
Q: What is the hybridization of carbon atom in benzene structure? Select one: O a spºd O b. sp O c. sp²…
A: Hybridization of any chemical compound is formed when the atomic orbitals fuse together to form the…
Q: 40. What is the approximate bond angle for the C-C-N bond in acetonitrile, CH3CN? A) 90° B) 109.5°…
A: The molecular shape and bond angle of a compound can be determined by the state of hybridization and…
Q: 2. Identify the "order" and "type" of the following halides of carbons that are sp3 hybridized.. Are…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: What is the set of valence orbitals on the carbon indicated with the arrow in this structure? All…
A:
Q: What is the hybridization of the central carbon atom of allene 1,2-propadiene
A: the hybridization of the central carbon atom of allene 1,2-propadiene is sp.
Q: Name: Cambapn Castills Date: 719/21 A. Mechanism of Hybridization # of Valence Atomic orbital of…
A: The compound given is BBr3.
Q: The number of sp2 hybridized carbon atom * in Ascorbic acid is: HO HO () 2 О 1 О з 4 ОН ОН О
A: sp2 hybridized carbon must be attached with a double bond.
Q: What is the hybridization on carbon in benzene? sp sp2 sp3 sp3d
A: Hybridization is the hypothetical mixing of atomic orbitals to form hybrid orbitals. The…
Q: Which of the labeled double bonds has trans geometry? A O Conly A & B A & C B only O A only B C * OH
A: Cis geometry - Same groups are on same side of the doubly bonded carbon atoms. Trans geometry - Same…
Q: 44. In the molecular orbital model of cyclobutadiene, how many T-antibonding molecular orbitals are…
A:
Q: What orbitals are used to form the labeled bonds in the following molecule? Of the labeled C—C…
A: 1--> C-H bond is formed due to overlap of sp2-s orbitals 2--> C-C bond is formed due to…
Q: What are the two kinds of s bonds found in benzene?
A: Benzene is an inorganic compound with the molecular formula C6H6 . It is an aromatic compound with a…
Q: Which structures do NOT have conjugated pi-bonds? O: I. II. I. IV. O A. I & II O B. I & II O C.I& IV
A: Please find your solution below : A conjugated system is a system that has alternate single and…
Q: 2. Name the following hydrocarbon. Predict the hybridization (sp³, sp2, o sp) in the asteroid…
A:
Q: Draw a carbon-based compound that is:…
A: Here we are asked to draw the structure of carbon- based compoundwith, sp, sp2 and sp3 hybridized
Q: .The hybridization of carbon atom in alkane is - 24 A-Sp³ 30+ 1. B - Sp³ 40 C − Sp3 2 ơ + 2 . A O BO…
A:
Q: Which C-C bond in the following molecule is formed from the overlap of an sp orbital and an sp?…
A:
Q: What is the hybridization around the carbon atom in the drawing below? нн C=C H H
A: The sp hybridization: If there is a triple bond around the atom, then it will be sp hybridization.…
Q: In the structure of cyclopentene, how many sp2 hybridized carbons are present? A) 1 B) 2 c) 4 D) 5
A:
Q: 4. structure and bonding) a. Draw the Lewis structure of l3-, including formal charges, and predict…
A: Valence electrons are combine together to form Lewis dot structure. Where shape of the molecule can…
Q: Which of the following is not true about o bonds o bonds are the 1st bonds made between two atoms…
A: Given, sigma bonds are the 1st bonds made between two atoms sigma bonds are formed from end to…
Q: The hybridization of the carbons in ethene are and the bond angles around each carbon are O sp3…
A: We have given that The hybridization of the carbons in ethene are and the bond…
Q: 1. Identify which type of hybridization the pointed element exhibit a. SR. b. sp2 C. sp3 d. sp4 2.…
A: Hybridization - it tells us the orbital contribution involved in bonding in a molecule(w.r.t that of…
Q: π 2) Show how the carbon p orbitals overlap to form the lowest energy л molecular orbital of 1,3-…
A:
Q: How many of the following molecules and ions have a bondorder of 1/2: H2, H2+, H2-, and He22 + ?(a)…
A: Bond order can be calculated by adding the number of electrons in the bonding orbital and…
Q: For SiSe2 which of the following best describes the lone pairs on the Selenium? they are found in…
A: Concept based on the hybridization.
Q: 9. Which of the following molecules has a nitrogen atom that is sp³ hybridized? Ex-100 Me N₂ B Me U…
A: In chemistry, hybridization is the idea of combining two atomic orbitals to create a new hybridized…
Q: lonization Energy Electronegativity Electron Affinity Polarizability None of these are correct e…
A: 6. * Electron affinity is amount of energy change when electron added to isolated gas atom to…
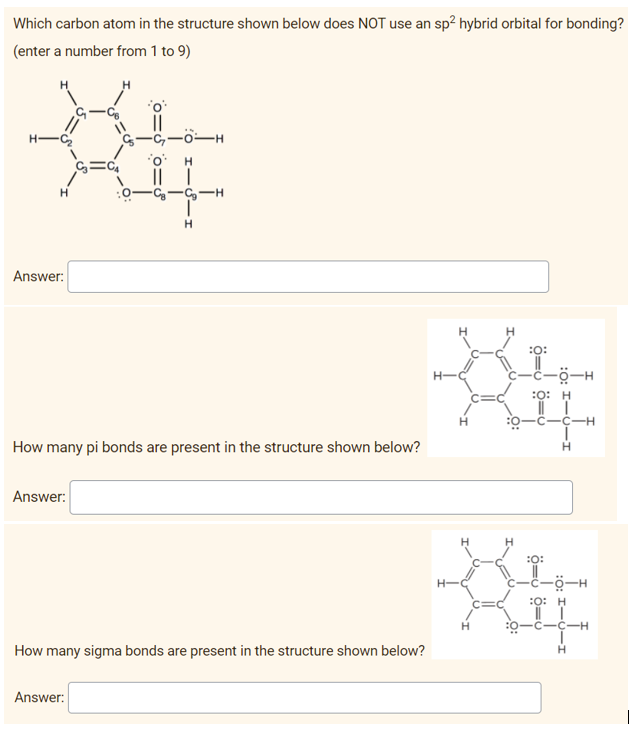
Q: Identify the carbon atom(s) in the structure shown that has (have) each of the following…
A: (a)sp3 hybridized carbon atoms in the molecule:
Q: What is the hybridization state of the central carbon atom in the structure below? A molecular…
A: Each carbon atom in allenes has a double bond with the carbon centres next to it. Cumulated dienes…
Q: _7 Assuming all of the following molecules are planar, which one can be labeled antiaromatic? HN IV…
A:
Q: What is the hybridization of the sigma bonding orbitals of the carbon atom?
A: The mixing of atomic orbitals which forms the hybridized orbitals of same characteristic and…
Q: • Consider the molecule shown below and answers the following questions. : Number of o bonds formed…
A: sp2 hybridization = 1 sigma + 1 pi sp hybridization = 1 sigma + 2 pi sp3 hybridization = 4 sigma (…
Q: 9. How many hybrid orbitals does a carbon atom have in an SP3 hybrid state? Draw the shape of the…
A: Hybridization is defined as the concept of mixing two or more atomic orbitals to give a new type of…
Q: ule is non-polar? :B HーN-H F: :Br-C :Br: O A :2-I
A: The molecules given are, A. HF B. CBr4 C. NH3 D. CHF3 Non polar molecule is ?
Q: (c) If we replace the hydrogen atoms in ammonia with the ethyl moieties (C2H5)3– N), there is no…
A: The formula of ammonia is NH3. Ethyl moieties are represented by -C2H5.
Q: The hybridization of carbon - 11 .atom in alkene is А 30 + 1 π. - B - 40 C-20+21. O O O
A:
Q: Does it matter which of the two sp3 hybrid orbitals are used tohold the two nonbonding electron…
A: Hybridization is the mixing of valence atomic orbitals to get equivalent hybridized orbitals that…
Q: Compare the charges of the molecules below. This time, I need you to identify the hybridization of…
A: Hybridisation means Molecular orbitals combine to form hybridised orbital,like in SP2,1-s ,2-p…
Q: Many reactions involve a change in hybridization of one or more atoms in the starting material. In…
A: When organic materials undergo chemical reaction they also undergo change in hybridization. This…
Q: Explain why a s bond formed by overlap of an s orbital with an sp3 orbital of carbon is stronger…
A: The S orbital is a sphere around the atomic nucleus. The P orbital is dumbbell-shaped.
Q: 8. Which of the following is nottrue of an sp hybridized carbon atom? a) One p orbital is present.…
A: Hybridization is the mixing of atomic orbitals having different energies to form hybrid orbitals of…
Q: 1. Chloroethane (CH3CH2Cl) is a topical anesthetic that boils at 12 °C. When liquid chloroethane is…
A: We are authorized to answer one question at a time, since you have not mentioned which question you…
Q: Problem attached
A: It is given that five structures and the structure of that compound need to be determined who has…
Q: 4. Describe the hybridization of each carbon atom in the following structure.
A: Given compound is : Describe the hybridization of each carbon atom in the following structure ?…
Q: How many single bonds can an sp2 hybridized carbon have? O A. 4 О вз O c. 2 O D. 1
A: Since you have posted multiple questions, we are entitled to answer the first only.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

- Consider the incomplete orbital representation of O2 , below right. a. Identify which lobes are hybrid orbitals (identify the type) and which lobes arep orbitals. b. Use dotted lines to show any bonds. c. Use up or down arrows to show electron occupation of each hybrid orbital or bond.Represent the bonding in each molecule or ion by drawing the orbitals (hybridized and unhybridized) of each atom in the bond. Label the σ and π bonds and label each bond by the orbitals that it is made from. For instance, the C–H bond in CH4 would be made from the overlap of: C (sp3) – H (1 s). You may draw the hybridized sigma orbitals as sticks and the unhybridized p-orbitals as lobes for clairity and ease. a. HONO b. CH3 CCH c. C3 H4 d. C2 O4 2-VISUAL SKILLS The isomers of retinal have the same number of atoms and bonds but differ in thespatial arrangement at one carbon-carbon double bond (C“C). In each isomer, circle that C“C. Lookingat the atoms around that bond, to what atoms do the terms cis (same side) and trans (opposite side) refer?
- 3) Combine any Atomic orbitals to form Molecular orbitals assuming they fulfill the phase and energy requirement. Construct MO diagram (include AOs, MOs, pictures of each, electrons, HOMO, LUMO, B.O., and comparative energies, etc.). REMOVE an ELECTRON to form CATION Please only do part 3. The first image shows the problemIdentify the hybridization of the labeled carbons in the molecule Remdisivir. Answer all labels 1 to 10.a.Determine the number of nodes and rank them in order of increasing energy. b.Draw the p atomic orbital contributions on each carbon atom (AO mixing pattern) that would give rise to the MO. c. Based on your answer to part (c) -determine whether each MO is overall bonding, nonbonding, or antibonding. Please explain steps
- pls help ASAP! “which term best describes the relationship between the following two molecules?”Using your model of butane (CH3CH2CH2CH3) , complete the following graph of the anglebetween the two Me groups vs. potential energy. a. Label each Newman projection of butane on the graph with the words staggered, eclipsed, gauche, and anti, as appropriate. (Note that some structures will have more than one label.) b. Draw a wedge and dash bond representation of butane in its lowest P.E. conformation.Consider any one of the four identical hybrid orbitals in the 109.5° set. a. What fraction of the clay in this hybrid orbital was originally red (carne from the 2s orbital)? b. What fraction of the clay in this hybrid orbital was originally green (carne from a 2porbital)? c. Explain the name “ s(1/4)p(3/4) hybrid orbital” for each of the four orbitals. d. In fact, each of the four hybrid orbitals in the 109.5° set is called an sp3 -hybrid orbital.Explain the name “ sp3 -hybrid orbital.”
- What are all of the types of orbital overlaps that occur in the above structure. p-p overlap sp²-sp overlap s-sp² overlap sp²-sp² overlap s-sp overlap sp-sp overlap s-s overlap ---------- In cumulene, what are the C=C=C and H−C−H ideal bond angles, respectively?Enter the C=C=C bond angle followed by the H−C−H bond angle separated by a comma (no spaces, no º symbol required).each reagant must have 3 or less carbons . one image is the directions and one is the question.Assign Re and Si face to an SP2 hybridized carbon (C=O or C=C) Can you explain how to get it and provide a example?

