Chemistry 10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
1 Chemical Foundations 2 Atoms, Molecules, And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding: General Concepts 9 Covalent Bonding: Orbitals 10 Liquids And Solids 11 Properties Of Solutions 12 Chemical Kinetics 13 Chemical Equilibrium 14 Acids And Bases 15 Acid-base Equilibria 16 Solubility And Complex Ion Equilibria 17 Spontaneity, Entropy, And Free Energy 18 Electrochemistry 19 The Nucleus: A Chemist's View 20 The Representative Elements 21 Transition Metals And Coordination Chemistry 22 Organic And Biological Molecules Chapter8: Bonding: General Concepts
Chapter Questions Section: Chapter Questions
Problem 1RQ: Distinguish between the terms electronegativity versus electron affinity, covalent bond versus ionic... Problem 2RQ: When an element forms an anion, what happens to the radius? When an element forms a cation, what... Problem 3RQ: Define the term lattice energy. Why, energetically, do ionic compounds form? Fig. 3-8 illustrates... Problem 4RQ: Explain how bond energies can be used to estimate E for a reaction. Why is this an estimate of E?... Problem 5RQ Problem 6RQ: Explain the terms resonance and delocalized electrons. When a substance exhibits resonance, we say... Problem 7RQ: Define formal charge and explain how to calculate it. What is the purpose of the formal charge?... Problem 9RQ: Give two requirements that should be satisfied for a molecule to be polar. Explain why CF4 and XeF4... Problem 10RQ: Consider the following compounds: CO2, SO2, KrF2, SO3, NF3, IF3, CF4, SF4, XeF4, PF5, IF5, and SCl6.... Problem 1ALQ: Explain the electronegativity trends across a row and down a column of the periodic table. Compare... Problem 2ALQ: The ionic compound AB is formed. The charges on the ions may be +1, 1; +2, 2; +3, 3; or even larger.... Problem 3ALQ Problem 4ALQ: The bond energy for a CH bond is about 413 kJ/mol in CH4 but 380 kJ/mol in CHBr3. Although these... Problem 5ALQ Problem 6ALQ: Which has the greater bond lengths: NO2 or NO3? Explain. Problem 7ALQ: The following ions are best described with resonance structures. Draw the resonance structures, and... Problem 9ALQ: The second electron affinity values for both oxygen and sulfur are unfavorable (positive). Explain. Problem 10ALQ: What is meant by a chemical bond? Why do atoms form bonds with each other? Why do some elements... Problem 11ALQ: Why are some bonds ionic and some covalent? Problem 12ALQ: How does a bond between Na and Cl differ from a bond between C and O? What about a bond between N... Problem 13ALQ: Arrange the following molecules from most to least polar and explain your order: CH4, CF2C12, CF2H2,... Problem 14ALQ: Does a Lewis structure tell which electron come from which atoms? Explain. Problem 15ALQ: True or false? In general, a large atom has a smaller electro-negativity. Explain. Problem 16ALQ: What is the central idea of the VSEPR model? Problem 17ALQ: In Section 8.13 of the text, the term effective pair is used. What does this term mean? Problem 20Q: Describe the type of bonding that exists in die F2(g) molecule. How does this type of bonding differ... Problem 21Q: Some plant fertilizer compounds are (NH4)2SO4, Ca3(PO4)2, K2O, P2O5, and KC1. Which of these... Problem 22Q: Some of the important properties of ionic compounds are as follows: i. low electrical conductivity... Problem 23Q: What is the electronegativity trend? Where does hydrogen fit into the electronegativity trend for... Problem 24Q: Give one example of a compound having a linear molecular structure that has an overall dipole moment... Problem 25Q: When comparing the size of different ions, the general radii trend discussed in Chapter 2 is usually... Problem 26Q: In general the higher the charge on the ions in an ionic compound, the more favorable the lattice... Problem 27Q: Combustion reactions of fossil fuels provide most of the energy needs of the world. Why do the... Problem 28Q: Which of the following statements is/are true? Correct the false statements. a. It is impossible to... Problem 29Q Problem 30Q: The molecules BF3, CF4, CO2, PF5, and SF6 are all nonpolar, even though they contain polar bonds.... Problem 31E: Without using Fig. 3-4, predict the order of increasing electronegativity in each of the following... Problem 32E: Without using Fig. 3-4, predict the order of increasing electronegativity in each of the following... Problem 33E: Without using Fig. 3-4, predict which bond in each of the following groups will be the most polar.... Problem 34E: Without using Fig. 3-4, predict which bond in each of the following groups will be the most polar.... Problem 35E Problem 36E Problem 37E: Which of the following incorrectly shows the bond polarity? Show the correct bond polarity for those... Problem 38E: Indicate the bond polarity (show the partial positive and partial negative ends) in the following... Problem 39E: Predict the type of bond (ionic, covalent, or polar covalent) one would expect to form between the... Problem 40E: List all the possible bonds that can occur between the elements P, Cs, O, and H. Predict the type of... Problem 41E: Hydrogen has an electronegativity value between boron and carbon and identical to phosphorus. With... Problem 42E: Rank the following bonds in order of increasing ionic character: NO, CaO, CF, BrBr, KF. Problem 43E: State whether or not each of the following has a permanent dipole moment. a. b. c. d. e. f. Problem 44E: The following electrostatic potential diagrams represent CH4, NH3, or H2O. Label each and explain... Problem 45E Problem 46E Problem 47E: Predict the empirical formulas of the ionic compounds formed from the following pairs of elements.... Problem 48E: Predict the empirical formulas of the ionic compounds formed from the following pairs of elements.... Problem 49E: Write electron configurations for a. the cations Mg2+, K+, and Al3+. b. the anions N3, O2, F, and... Problem 50E: Write electron configurations for a. the cations Sr2+, Cs+, In+, and Pb2+. b. the anions P3, S2, and... Problem 51E: Which of the following ions have noble gas electron configurations? a. Fe2+, Fe3+, Sc3+, Co3+ b.... Problem 52E: What noble gas has the same electron configuration as each of the ions in the following compounds?... Problem 53E: Give the formula of a negative ion that would have the same number of electrons as each of the... Problem 54E Problem 55E: Give three ions that are isoelectronic with neon. Place these ions in order of increasing size. Problem 56E: Consider the ions Sc3+, Cl, K+, Ca2+, and S2. Match these ions to the following pictures that... Problem 57E Problem 58E: For each of the following groups, place the atoms and/or ions in order of decreasing size. a. V,... Problem 59E: Which compound in each of the following pairs of ionic substances has the most negative lattice... Problem 60E: Which compound in each of the following pairs of ionic substances has the most negative lattice... Problem 61E: Use the following data for potassium chloride to estimate E for the reaction: K(s)+12Cl2(g)KCL(s)E=?... Problem 62E: Use the following data for magnesium fluoride to estimate E for the reaction: Mg(s)+F2(g)MgF2(s)E=?... Problem 63E: Consider the following energy changes: E(kJ/mol) Mg(g) Mg+(g) + e 735 Mg+ (g) Mg2+(g) + e 1445... Problem 64E: Compare the electron affinity of fluorine to the ionization energy of sodium. Does the process of an... Problem 65E Problem 66E: Use the following data (in kJ/mol) to estimate E for the reaction S(g)+e S2(g). Include an estimate... Problem 67E: Rationalize the following lattice energy values: Compound Lattice Energy (kJ/mol) CaSe 2862 Na2Se... Problem 68E: The lattice energies of FeCl3, FeCl2, and Fe2O3 are (in no particular order) 2631, 5359, and 14,774... Problem 69E: Use bond energy values (Table 3-3) to estimate E for each of the following reactions in the gas... Problem 70E: Use bond energy values (Table 3-3) to estimate E for each of the following reactions. a. b. Problem 71E Problem 72E: Acetic acid is responsible for the sour taste of vinegar. It can be manufactured using the following... Problem 73E: Use bond energies to predict E for the following reaction: H2S(g)+3F2(g)SF4(g)+2HF(g) Problem 74E: The major industrial source of hydrogen gas is by the following reaction: CH4(g)+H2O(g)CO(g)+3H2(g)... Problem 75E: Use bond energies to estimate E for the combustion of one mole of acetylene:... Problem 76E Problem 77E Problem 78E: Consider the following reaction: A2+B22AB E = 285kJ The bond energy for A2 is one-half the amount of... Problem 79E: Compare your answers from parts a and b of Exercise 69 with H values calculated for each reaction... Problem 80E: Compare your answers from Exercise 72 to the H values calculated from standard enthalpies of... Problem 81E: The standard enthalpies of formation for S(g), F(g), SF4(g), and SF6(g) are + 278.8, + 79.0, 775,... Problem 82E: Use the following standard enthalpies of formation to estimate the NH bond energy in ammonia: N(g),... Problem 83E: The standard enthalpy of formation for N2H2(g) is 95.4 kJ/mol. Use this and the data in Exercise 82... Problem 84E: The standard enthalpy of formation for NO(g) is 90. kJ/mol. Use this and the values for the O=O and... Problem 85E: Write Lewis structures that obey the octet rule (duet rule for H) for each of the following... Problem 86E: Write Lewis structures that obey the octet rule (duet rule for H) for each of the following... Problem 87E: Write Lewis structures that obey the octet rule for each of the following molecules. a. CCl4 b. NCl3... Problem 88E: Write Lewis structures that obey the octet rule for each of the following molecules and ions. (In... Problem 89E: One type of exception to the octet rule are compounds with central atoms having fewer than eight... Problem 90E: Lewis structures can be used to understand why some molecules react in certain ways. Write the Lewis... Problem 91E: The most common exceptions to the octet rule are compounds or ions with central atoms having more... Problem 92E Problem 93E: Write Lewis structures for the following. Show all resonance structures where applicable. a. NO2,... Problem 94E Problem 95E: Benzene (C6H6) consists of a six-membered ring of carbon atoms with one hydrogen bonded to each... Problem 96E: Borazine (B3N3H6) has often been called inorganic benzene. Write Lewis structures for borazine.... Problem 97E: An important observation supporting the concept of resonance in the localized electron model was... Problem 98E: Consider the following bond lengths: CO143pmC9O123 pmC:O109 pm In the Co32 ion, all three C8O bonds... Problem 99E: A toxic cloud covered Bhopal, India, in December 1984 when water leaked into a tank of methyl... Problem 103E Problem 104E Problem 105E: Write Lewis structures that obey the octet rule for the following species. Assign the formal charge... Problem 106E: Write Lewis structures for the species in Exercise 99 that involve minimum formal charges. Problem 108E: Oxidation of the cyanide ion produces the stable cyanate ion, OCN. The fulminate ion, CNO, on the... Problem 109E: When molten sulfur reacts with chlorine gas, a vile-smelling orange liquid forms that has an... Problem 110E: Carbon and sulfur form compounds with each other with the formulas CS2 and C3S2. Draw a Lewis... Problem 112E Problem 113E: Predict the molecular structure and bond angles for each molecule or ion in Exercises 87 and 93. a.... Problem 114E: Predict die molecular structure and bond angles for each molecule or ion in Exercises 88 and 94. a.... Problem 115E: There are several molecular structures based on the trigonal bipyramid geometry (see Table 8.9).... Problem 116E: Two variations of the octahedral geometry (see Table 8.7) are illustrated below. Which of the... Problem 117E Problem 118E: Consider the molecular structures illustrated in the previous exercise. For each structure, give an... Problem 119E: Predict the molecular structure (including bond angles) for each of the following. a. SeO3 b. SeO2 Problem 120E: Predict the molecular structure (including bond angles) for each of the following. a. PCl3 b. SCl2... Problem 121E: Predict the molecular structure (including bond angles) for each of the following. (See Exercises... Problem 122E: Predict the molecular structure (including bond angles) for each of the following. (See Exercises... Problem 123E Problem 124E: Which of the molecules in Exercise 120 have net dipole moments (are polar)? Problem 125E: Which of the molecules in Exercise 121 have net dipole moments (are polar)? Problem 126E: Which of the molecules in Exercise 122 have net dipole moments (are polar)? Problem 127E: Write Lewis structures and predict the molecular structures of the following. (See Exercises 115 and... Problem 128E: Write Lewis structures and predict whether each of the following is polar or nonpolar. a. HOCN... Problem 129E: Consider the following Lewis structure where E is an unknown element: What are some possible... Problem 130E: Consider the following Lewis structure where E is an unknown element: What are some possible... Problem 131E: Two different compounds exist having the formula N2F2. One compound is polar whereas the other is... Problem 132E: Two different compounds have the formula XeF2Cl2. Write Lewis structures for these two compounds,... Problem 133AE: Arrange the following in order of increasing radius and increasing ionization energy. a. N+, N, N b.... Problem 134AE: For each of the following, write an equation that corresponds to the energy given. a. lattice energy... Problem 135AE: Use bond energies (table 3-3), values of electron affinities (table 2-7), and the ionization energy... Problem 136AE: Write Lewis structures for CO32, HCO3, and H2CO3. When acid is added to an aqueous solution... Problem 137AE: Which member of the following pairs would you expect to be more energetically stable? Justify each... Problem 138AE: What do each of the following sets of compounds/ions have in common? a. SO3, NO3, CO32 b. O3, SO2,... Problem 139AE Problem 140AE: Although both Br3 and I3 ions are known, the F3 ion has not been observed. Explain. Problem 142AE Problem 144AE: Which of the following molecules have not dipole moments? For the molecules that are polar, indicate... Problem 145AE Problem 146AE: Look up the energies for the bonds in CO and N2. Although the bond in CO is stronger, CO is... Problem 147CWP: Classify the bonding in each of the following molecules as ionic, polar covalent, or nonpolar... Problem 148CWP: List the bonds PCl, PF, OF, and SiF from least polar to most polar. Problem 149CWP: Arrange the atoms and/or ions in the following groups in order of decreasing size. a. O, O, O2 b.... Problem 150CWP: Use the following data to estimate E for the reaction: Ba(s)+Br2(g)BaBr2(s)E=? Lattice energy 1985... Problem 151CWP: Use bond energy values to estimate E for the following gas phase reaction: C2H4+H2O2CH2OHCH2OH Problem 152CWP: Which of the following compounds or ions exhibit resonance? a. O3 b. CNO c. Asl3 d. CO32 e. AsF3 Problem 153CWP: The formulas of several chemical substances are given in the table below. For each substance in the... Problem 154CWP: Predict the molecular structure, bond angles, and polarity (has a net dipole moment or has no net... Problem 155CP: Use Coulombs Jaw, V=Q1Q240r=2.311019Jnm(Q1Q2r) to calculate the energy of interaction, V. for the... Problem 156CP Problem 157CP: Calculate the standard heat of formation of the compound ICl(g) at 25C. (Hint: Use Table 8.5 and... Problem 158CP: Given the following information: Energy of sublimation of Li(s) = 166 kJ/mol Bond energy of HCl =... Problem 159CP Problem 160CP: Think of forming an ionic compound as three steps (this is a simplification, as with all models):... Problem 161CP: The compound NF3 is quite stable, but NCl3, is very unstable (NCl3, was first synthesized in 1811 by... Problem 162CP: Three processes that have been used for the industrial manufacture of acrylonitrile (CH2CHCN), an... Problem 163CP: The compound hexaazaisowurtzitane is one of the highest-energy explosives known (C E News, Jan. 17,... Problem 164CP: Many times extra stability is characteristic of a molecule or ion in which resonance is possible.... Problem 165CP: The study of carbon-containing compounds and their properties is called organic chemistry. Besides... Problem 166CP: Draw a Lewis structure for the N, N-dimethylformamide molecule. The skeletal structure is Various... Problem 167CP Problem 168CP: Consider the following computer-generated model of caffeine. Draw a Lewis structure for caffeine in... Problem 169CP: Cholesterol (C27H46O) has the following structure: In such shorthand structures, each point where... Problem 171IP: A compound, XF5, is 42.81% fluorine by mass. Identify the element X. What is the molecular structure... Problem 173IP: Identify the following elements based on their electron configurations and rank them in order of... Problem 1RQ: Distinguish between the terms electronegativity versus electron affinity, covalent bond versus ionic...
Related questions
Concept explainers
13) The average C−C bond dissociation energy (D) is 350 kJ/mol and the average C=C bond dissociation energy is 728 kJ/mol. Based on these values, which is stronger: a σ or a π bond?
Transcribed Image Text: 378
π
965
weaker
O
265
stronger
28
1078
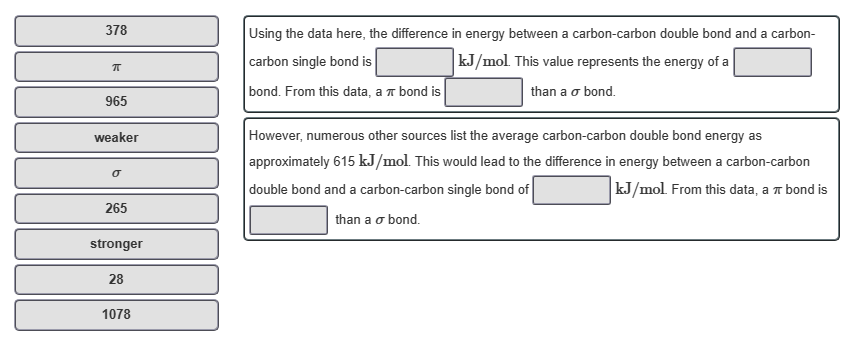
Using the data here, the difference in energy between a carbon-carbon double bond and a carbon-
carbon single bond is
kJ/mol. This value represents the energy of a
bond. From this data, a π bond is
than a o bond.
However, numerous other sources list the average carbon-carbon double bond energy as
approximately 615 kJ/mol. This would lead to the difference in energy between a carbon-carbon
double bond and a carbon-carbon single bond of
kJ/mol. From this data, a π bond is
than a o bond.
Formula Formula Bond dissociation energy (BDE) is the energy required to break a bond, making it an endothermic process. BDE is calculated for a particular bond and therefore consists of fragments such as radicals since it undergoes homolytic bond cleavage. For the homolysis of a X-Y molecule, the energy of bond dissociation is calculated as the difference in the total enthalpy of formation for the reactants and products. X-Y → X + Y BDE = Δ H f X + Δ H f Y – Δ H f X-Y where, ΔHf is the heat of formation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step 1: Description of the question
VIEW
Step by step
Solved in 3 steps with 5 images