39) One of the buffers that contribute to pH stability in human blood is carbonic acid (H₂CO3). Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO3-) and a hydrogen ion (H+). (See figure.) H₂CO3 Carbonic acid HCO3 Bicarbonate ion H+ Hydrogen ion If the pH of blood drops, one would expect A) a decrease in the concentration of H2CO3 and an increase in the concentration of HCO3- B) the concentration of bicarbonate ions (HCO3-) to increase C) the HCO3- to act as a base and remove excess H+ by the formation of H2CO3 D) the HCO3- to act as an acid and remove excess H+ by the formation of H₂CO3 Answer: C
39) One of the buffers that contribute to pH stability in human blood is carbonic acid (H₂CO3). Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO3-) and a hydrogen ion (H+). (See figure.) H₂CO3 Carbonic acid HCO3 Bicarbonate ion H+ Hydrogen ion If the pH of blood drops, one would expect A) a decrease in the concentration of H2CO3 and an increase in the concentration of HCO3- B) the concentration of bicarbonate ions (HCO3-) to increase C) the HCO3- to act as a base and remove excess H+ by the formation of H2CO3 D) the HCO3- to act as an acid and remove excess H+ by the formation of H₂CO3 Answer: C
Biology (MindTap Course List)
11th Edition
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Chapter2: Atoms And Molecules: The Chemical Basis Of Life
Section2.6: Acids, Bases, And Salts
Problem 1C
Related questions
Question
explain step by step why the correct answer is C
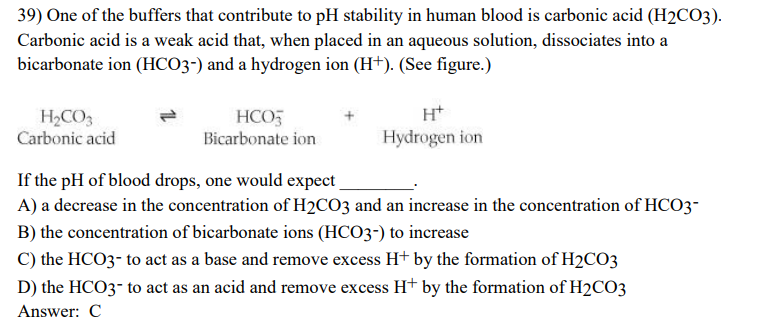
Transcribed Image Text:39) One of the buffers that contribute to pH stability in human blood is carbonic acid (H₂CO3).
Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a
bicarbonate ion (HCO3-) and a hydrogen ion (H+). (See figure.)
H₂CO3
Carbonic acid
HCO3
Bicarbonate ion
H+
Hydrogen ion
If the pH of blood drops, one would expect
A) a decrease in the concentration of H₂CO3 and an increase in the concentration of HCO3-
B) the concentration of bicarbonate ions (HCO3-) to increase
C) the HCO3- to act as a base and remove excess H+ by the formation of H2CO3
D) the HCO3- to act as an acid and remove excess H+ by the formation of H₂CO3
Answer: C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College