4 Part A How would you prepare an NaHCO3 - Na2CO3 (Kai (H2CO3) = 4.3-107, Ka2 (H2CO3) = 5.6 10-11) buffer solution that has pH=10.39? Express your answer using two significant figures. Make the Na2CO3 concentration O ΜΕ ΑΣΦ Submit Previous Answers Request Answer X Incorrect; Try Again; 3 attempts remaining ? times the concentration of NaHCO3. Provide Feedback
4 Part A How would you prepare an NaHCO3 - Na2CO3 (Kai (H2CO3) = 4.3-107, Ka2 (H2CO3) = 5.6 10-11) buffer solution that has pH=10.39? Express your answer using two significant figures. Make the Na2CO3 concentration O ΜΕ ΑΣΦ Submit Previous Answers Request Answer X Incorrect; Try Again; 3 attempts remaining ? times the concentration of NaHCO3. Provide Feedback
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 127QRT
Related questions
Question
Payalben
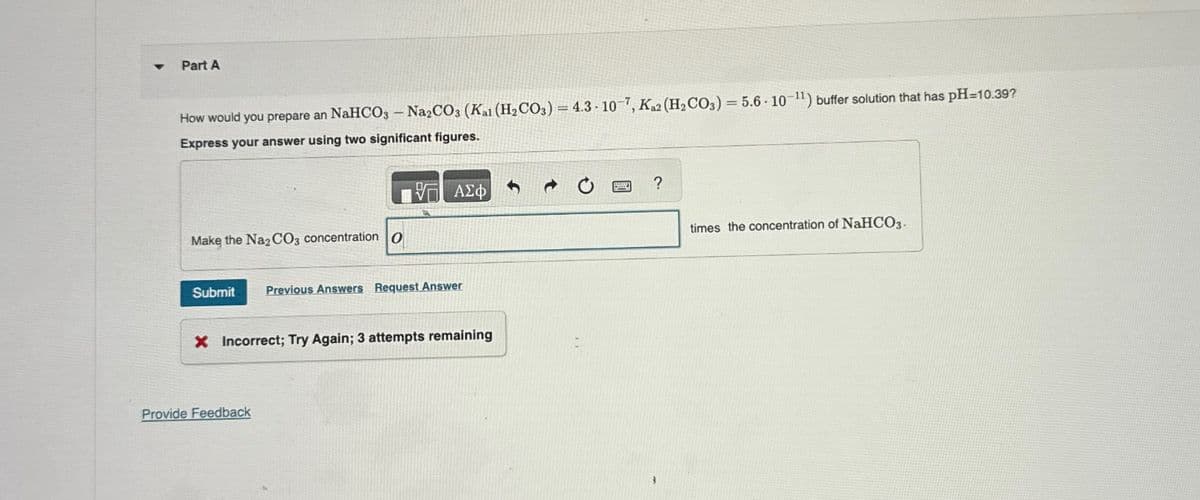
Transcribed Image Text:4
Part A
How would you prepare an NaHCO3 - Na2CO3 (Kai (H2CO3) = 4.3-107, Ka2 (H2CO3) = 5.6 10-11) buffer solution that has pH=10.39?
Express your answer using two significant figures.
Make the Na2CO3 concentration O
ΜΕ ΑΣΦ
Submit Previous Answers Request Answer
X Incorrect; Try Again; 3 attempts remaining
?
times the concentration of NaHCO3.
Provide Feedback
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning