4. A lightbulb contains 0.0753 g of helium gas with a pressure of 189 torr at 22.0 °C. The light bulb is then turned on and, after a period of time, the temperature rises to 279 °C. Calculate the final pressure inside the lightbulb.
4. A lightbulb contains 0.0753 g of helium gas with a pressure of 189 torr at 22.0 °C. The light bulb is then turned on and, after a period of time, the temperature rises to 279 °C. Calculate the final pressure inside the lightbulb.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter4: Introduction To Gases
Section: Chapter Questions
Problem 4.1TC: Target check For each of the macroscopic charcateristics unique to the gas phase of matter described...
Related questions
Question
Please help with number 4. Thank you
Transcribed Image Text:Name:
Peer Leader:
Date:
For all problems involving calculations, show your work. Carry the units through each
calculation and follow significant figure rules.
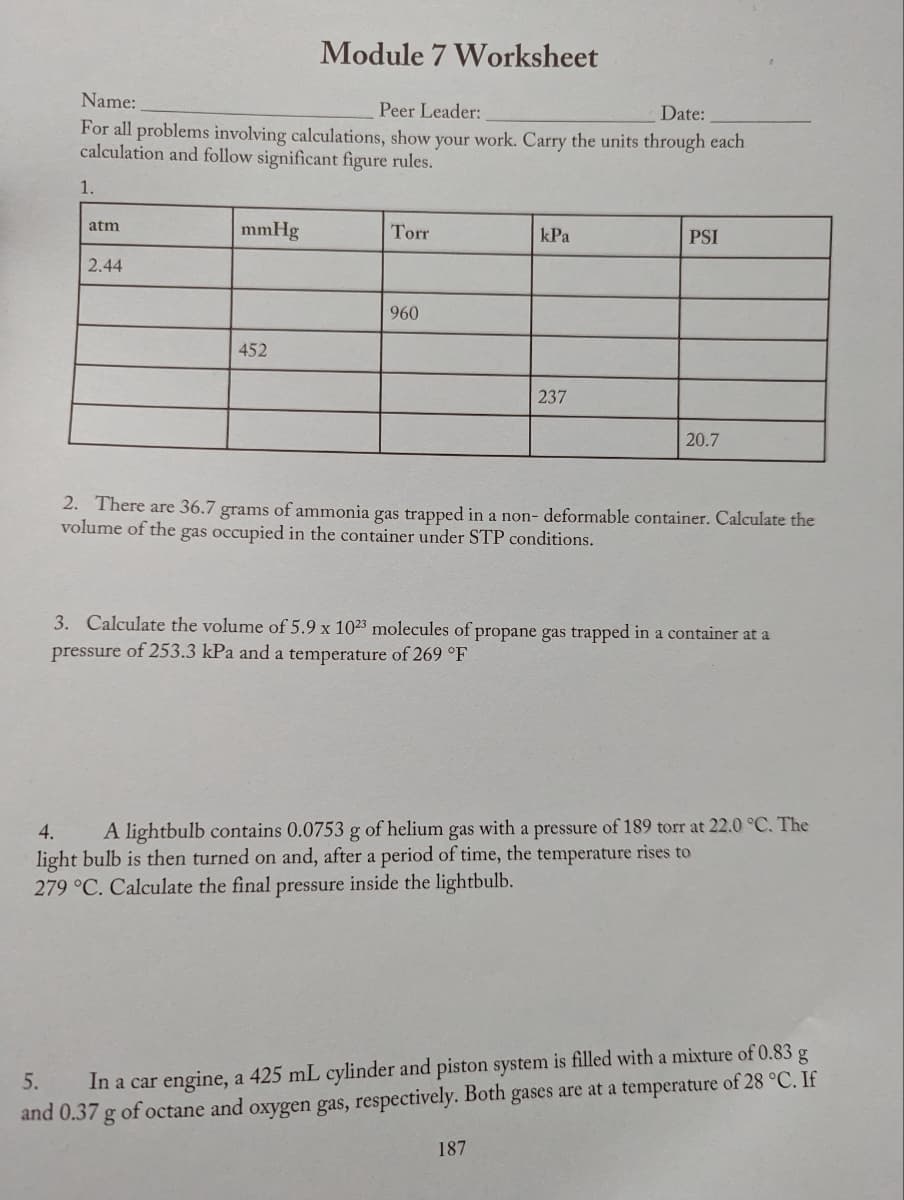
1.
atm
2.44
mmHg
Module 7 Worksheet
452
Torr
960
kPa
237
PSI
20.7
2. There are 36.7 grams of ammonia gas trapped in a non- deformable container. Calculate the
volume of the gas occupied in the container under STP conditions.
187
3. Calculate the volume of 5.9 x 1023 molecules of propane gas trapped in a container at a
pressure of 253.3 kPa and a temperature of 269 °F
4.
A lightbulb contains 0.0753 g of helium gas with a pressure of 189 torr at 22.0 °C. The
light bulb is then turned on and, after a period of time, the temperature rises to
279 °C. Calculate the final pressure inside the lightbulb.
5.
In a car engine, a 425 mL cylinder and piston system is filled with a mixture of 0.83 g
and 0.37 g of octane and oxygen gas, respectively. Both gases are at a temperature of 28 °C. If
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning