4. a) The boiling point of methyl acetate is 58° C and the boiling point of propanoic acid is 141° C. How do you explain the difference? H₂C OCH3 Methyl Acetate b) Propanol has a boiling point of 97° C and acetic acid has a boiling point of 118° C. How do you explain the difference? H3C Propanol H₂C OH Propanoic Acid OH H₂C OH Acetic Acid b)
4. a) The boiling point of methyl acetate is 58° C and the boiling point of propanoic acid is 141° C. How do you explain the difference? H₂C OCH3 Methyl Acetate b) Propanol has a boiling point of 97° C and acetic acid has a boiling point of 118° C. How do you explain the difference? H3C Propanol H₂C OH Propanoic Acid OH H₂C OH Acetic Acid b)
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter14: Alcohols, Ethers, And Thiols
Section: Chapter Questions
Problem 14.48P: 14-48 Explain why methanethiol, CH3SH, has a lower boiling point (6°C) than methanol, CH3OH (65°C),...
Related questions
Question
Can you please give an explanation for question 4? Thank you
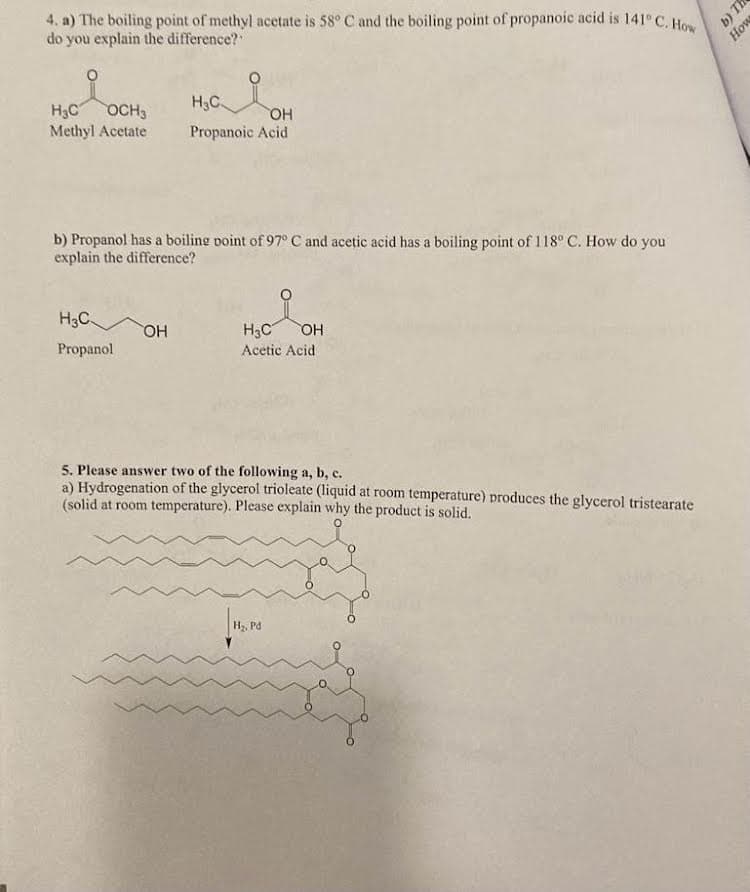
Transcribed Image Text:4. a) The boiling point of methyl acetate is 58° C and the boiling point of propanoic acid is 141° C. How
do you explain the difference?
H₂C OCH3
Methyl Acetate
b) Propanol has a boiling point of 97° C and acetic acid has a boiling point of 118° C. How do you
explain the difference?
H3C
Propanol
H₂C
OH
Propanoic Acid
OH
H₂C OH
Acetic Acid
5. Please answer two of the following a, b, c.
a) Hydrogenation of the glycerol trioleate (liquid at room temperature) produces the glycerol tristearate
(solid at room temperature). Please explain why the product is solid.
H₂, Pd
b) T
How
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
