4. Below is a simplified mechanism for the epoxidation of styrene via two transition states (TS-1 and TS-2) to give the two enantiomeric products ((R) styrene oxide and (S) styrene oxide). H t-Bu- -t-Bu `t-Bu H., t-Bu (R) styrene oxide TS 1 + t-Bu styrene `t-Bu Mn t-Bu- -t-Bu Mn=0 t-Bu t-Bu H. --Bu (S) styrene oxide t-Bu TS 2 a) Circle which of the following best describes the relationship between TS-1 and TS-2? i) Regioisomeri; ii) Diastereomeric; iii) Enantiomeric; iv) Isomeric; v) Mesomeric.
4. Below is a simplified mechanism for the epoxidation of styrene via two transition states (TS-1 and TS-2) to give the two enantiomeric products ((R) styrene oxide and (S) styrene oxide). H t-Bu- -t-Bu `t-Bu H., t-Bu (R) styrene oxide TS 1 + t-Bu styrene `t-Bu Mn t-Bu- -t-Bu Mn=0 t-Bu t-Bu H. --Bu (S) styrene oxide t-Bu TS 2 a) Circle which of the following best describes the relationship between TS-1 and TS-2? i) Regioisomeri; ii) Diastereomeric; iii) Enantiomeric; iv) Isomeric; v) Mesomeric.
Chapter11: Reactions Of Alkyl Halides: Nucleophilic Substitutions And Eliminations
Section11.SE: Something Extra
Problem 54AP
Related questions
Question
Need help with part (a). Thank you :)
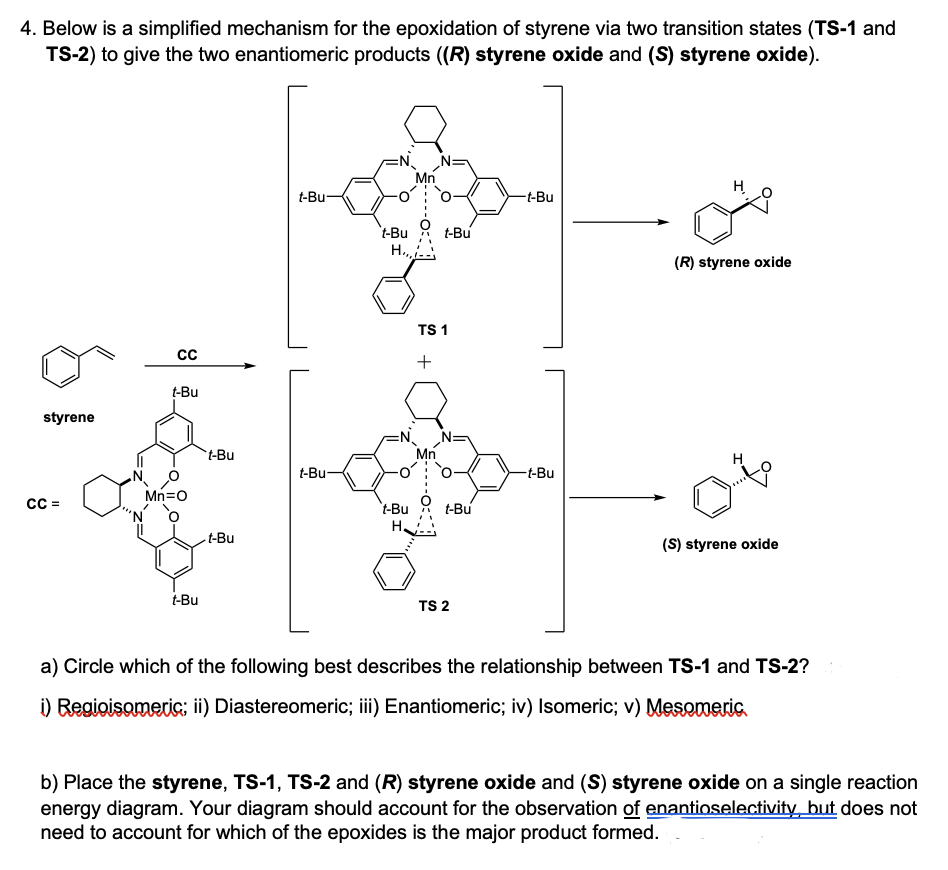
Transcribed Image Text:4. Below is a simplified mechanism for the epoxidation of styrene via two transition states (TS-1 and
TS-2) to give the two enantiomeric products ((R) styrene oxide and (S) styrene oxide).
Mn
H
t-Bu-
-t-Bu
't-Bu
t-Bu
H.,
(R) styrene oxide
TS 1
+
t-Bu
styrene
t-Bu
Mn
t-Bu-
-t-Bu
Mn=o
t-Bu
t-Bu
-t-Bu
(S) styrene oxide
t-Bu
TS 2
a) Circle which of the following best describes the relationship between TS-1 and TS-2?
i) Regioisomeri; ii) Diastereomeric; iii) Enantiomeric; iv) Isomeric; v) Mesomeric.
b) Place the styrene, TS-1, TS-2 and (R) styrene oxide and (S) styrene oxide on a single reaction
energy diagram. Your diagram should account for the observation of enantioselectivity but does not
need to account for which of the epoxides is the major product formed.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you



