4. Calculate the change in pH that results from adding 25 mL of 0.010F HCI to 500ml buffer solution which is 0.25M ammonia and 0.15M ammonium chloride.
4. Calculate the change in pH that results from adding 25 mL of 0.010F HCI to 500ml buffer solution which is 0.25M ammonia and 0.15M ammonium chloride.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.63P
Related questions
Question
pls answer the fourth item
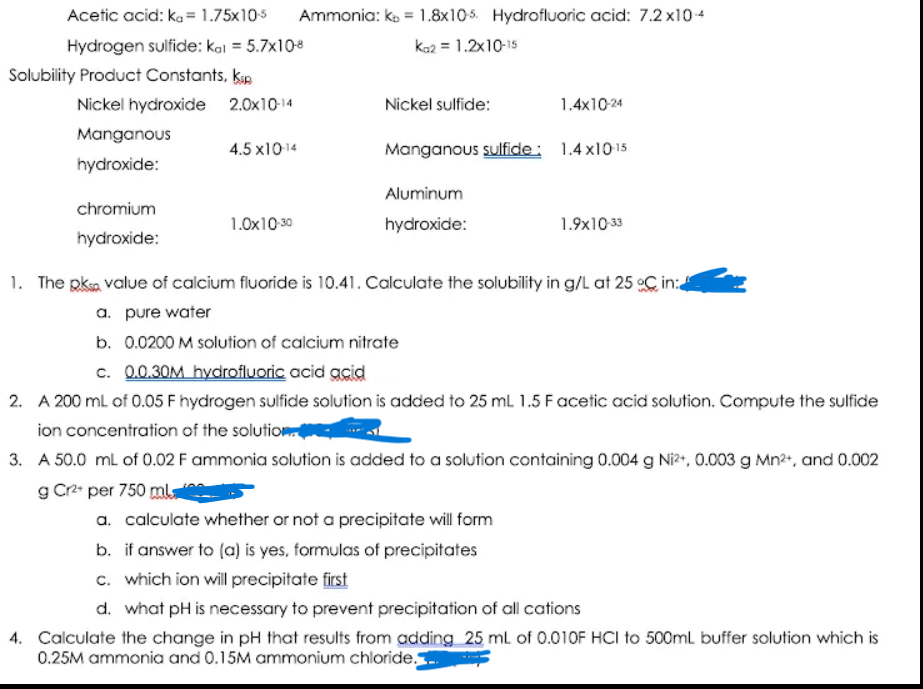
Transcribed Image Text:Acetic acid: ka = 1.75x105
Ammonia: ko = 1.8x105. Hydrofluoric acid: 7.2 x104
Hydrogen sulfide: kal = 5.7x108
kaz = 1.2x10-15
Solubility Product Constants, kap
Nickel hydroxide 2.0x10-14
Nickel sulfide:
1.4x10-24
Manganous
4.5 x1014
Manganous sulfide: 1.4 x1015
hydroxide:
Aluminum
chromium
1.0x10 30
hydroxide:
1.9x10 33
hydroxide:
1. The pksa value of calcium fluoride is 10.41. Calculate the solubility in g/L at 25 oC in:
a. pure water
b. 0.0200 M solution of calcium nitrate
c. 0.0.30M hydrofluoric acid acid
2. A 200 ml of 0.05 F hydrogen sulfide solution is added to 25 mL 1.5 Facetic acid solution. Compute the sulfide
ion concentration of the solution
3. A 50.0 ml of 0.02 F ammonia solution is added to a solution containing 0.004 g Ni?, 0.003 g Mn2+, and 0.002
g Cr2 per 750 mla
a. calculate whether or not a precipitate will form
b. if answer to (a) is yes, formulas of precipitates
c. which ion will precipitate first
d. what pH is necessary to prevent precipitation of all cations
4. Calculate the change in pH that results from adding 25 ml of 0.010F HCI to 500ml buffer solution which is
0.25M ammonia and 0.15M ammonium chloride.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 7 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning