Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter17: Applications Of Infrared Spectrometry
Section: Chapter Questions
Problem 17.9QAP
Related questions
Question
100%
SHOW COMPLETE SOLUTIONS
Q3. Which isolate has A260/280 ratio of above 2.0?
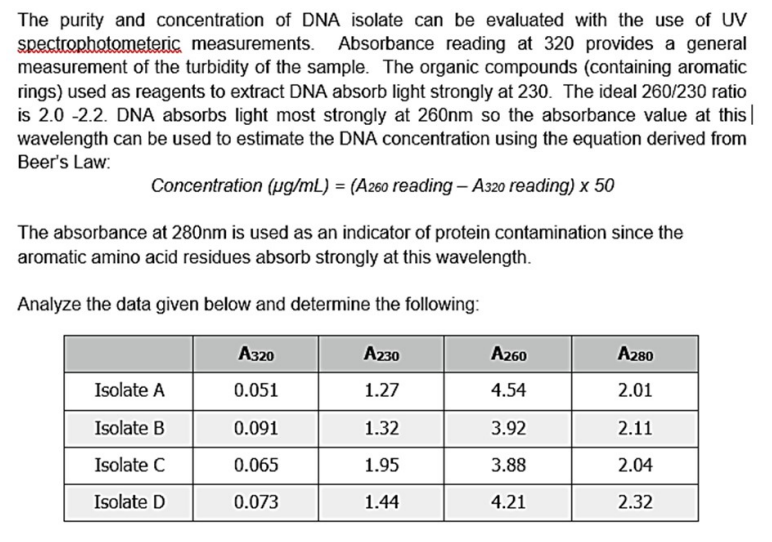
Transcribed Image Text:The purity and concentration of DNA isolate can be evaluated with the use of UV
spectrophotometeric measurements. Absorbance reading at 320 provides a general
measurement of the turbidity of the sample. The organic compounds (containing aromatic
rings) used as reagents to extract DNA absorb light strongly at 230. The ideal 260/230 ratio
is 2.0 -2.2. DNA absorbs light most strongly at 260nm so the absorbance value at this
wavelength can be used to estimate the DNA concentration using the equation derived from
Beer's Law:
Concentration (µg/mL) = (A260 reading - A320 reading) x 50
The absorbance at 280nm is used as an indicator of protein contamination since the
aromatic amino acid residues absorb strongly at this wavelength.
Analyze the data given below and determine the following:
Isolate A
Isolate B
Isolate C
Isolate D
A320
0.051
0.091
0.065
0.073
A230
1.27
1.32
1.95
1.44
A260
4.54
3.92
3.88
4.21
A280
2.01
2.11
2.04
2.32
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
