4. Compare the sum of the Cu-Zn and the Zn-Mg cell potentials with the Cu-Mg cell potential. What can you conclude? 5. What effect, if any, would varying the concentration of both the aqueous species have on the cell potentials? What about varying the size of the electrodes?
4. Compare the sum of the Cu-Zn and the Zn-Mg cell potentials with the Cu-Mg cell potential. What can you conclude? 5. What effect, if any, would varying the concentration of both the aqueous species have on the cell potentials? What about varying the size of the electrodes?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 135CWP: Consider a galvanic cell based on the following half-reactions: a. What is the expected cell...
Related questions
Question

Transcribed Image Text:4. Compare the sum of the Cu-Zn and the Zn-Mg cell potentials with the Cu-Mg cell potential.
What can you conclude?
5. What effect, if any, would varying the concentration of both the aqueous species have on the
cell potentials? What about varying the size of the electrodes?
144 EXPERIMENT 12
PA
K
(4
Transcribed Image Text:Name
EXPERIMENT 12
ELECTROCHEMISTRY: GALVANIC CELLS
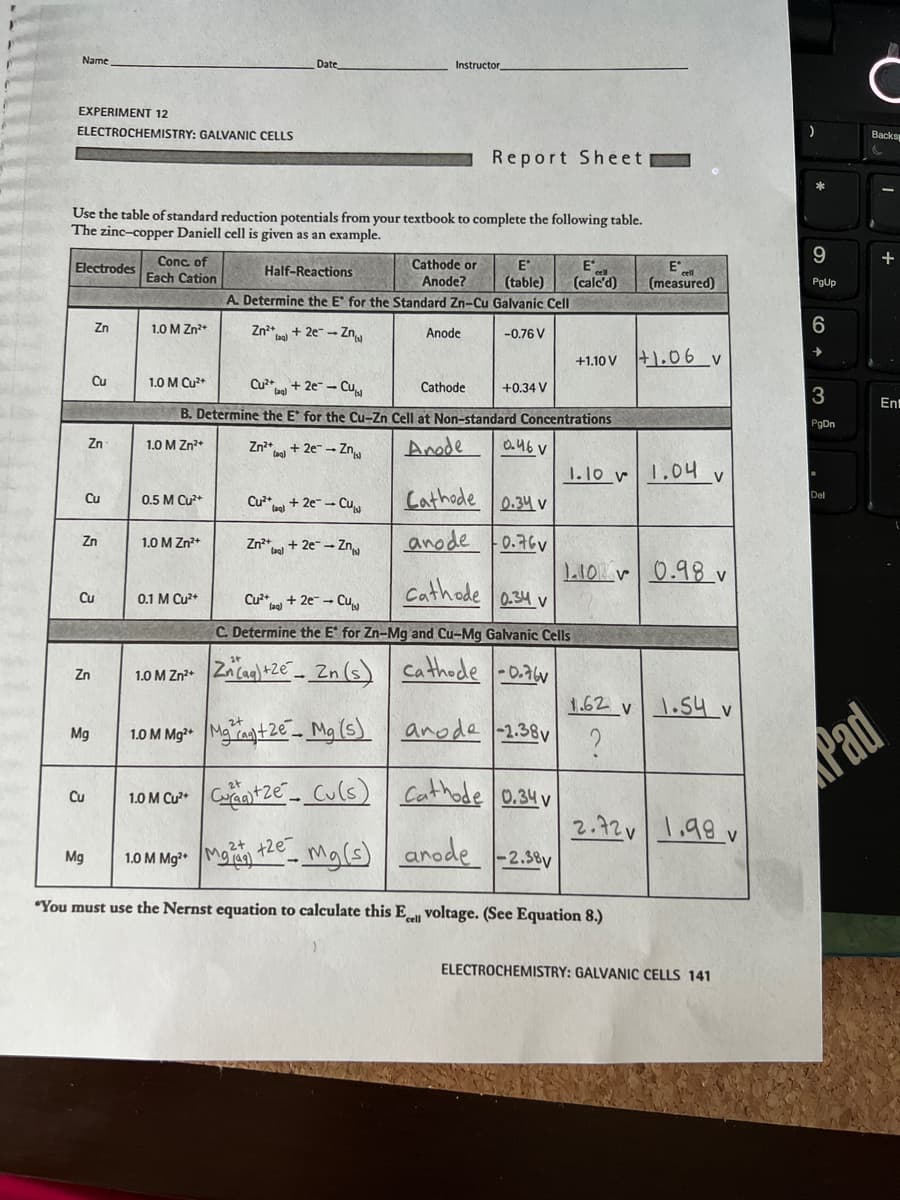
Electrodes
Use the table of standard reduction potentials from your textbook to complete the following table.
The zinc-copper Daniell cell is given as an example.
Cu
Zn
Zn
Zn
Cu
Cu
Zn
Mg
Cu
Mg
Conc. of
Each Cation
1.0 M Zn²+
1.0 M Cu²+
0.5 M Cu²+
1.0 M Zn²+
Date
0.1 M Cu²+
Zn²+ + 2e-Zn
(ag)
Instructor
Cu²+2e-Cu
B. Determine the E* for the Cu-Zn Cell at Non-standard Concentrations
Anode
0.46 V
1.0 M Zn²+
Cu²+ + 2e-Cu
Half-Reactions
A. Determine the E for the Standard Zn-Cu Galvanic Cell
Zn²+ + 2e-Zn
-0.76 V
Zn²+ + 2e- → Zn
Cathode or
Anode?
+2e
Report Sheet
Anode
E
E
(table) (calc'd)
Cathode
+0.34 V
Cathode 0.34 v
anode 0.76
1.0 M Zn²+ Zn (aq) +2e-Zn (s)
1.0 M Mg²+Mga+2e Mg (s)
1.0 M Cu²+C+Ze
Cu(s)
1.0 M Mg²+ M
Mg(s)
"You must use the Nernst equation to calculate this Ecell voltage. (See Equation 8.)
Cathode 0.34 v
Cu²+ + 2e → Cu
C. Determine the E for Zn-Mg and Cu-Mg Galvanic Cells
Cathode -0.76
anode -2.38v
+1.10 V 1.06 v
Cathode 0.34v
arode -2.38v
1.10 1.04 v
E
(measured)
1.10 0.98 v
1.62 v
?
1.54 v
2.72 1.98v
ELECTROCHEMISTRY: GALVANIC CELLS 141
9
PgUp
6
3
PgDn
Del
C
Backs
Pad
+
Ent
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax